Nucleic acid structure and expression carrier for enhancing recombinant protein production and mass-production method of recombinant protein
A technology of nucleic acid constructs and recombinant vectors, applied in hemoglobin/myoglobin, using vectors to introduce foreign genetic material, biochemical equipment and methods, etc., can solve problems such as difficulties, achieve promotional efficiency, shorten time, and facilitate cultivation The effect of the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
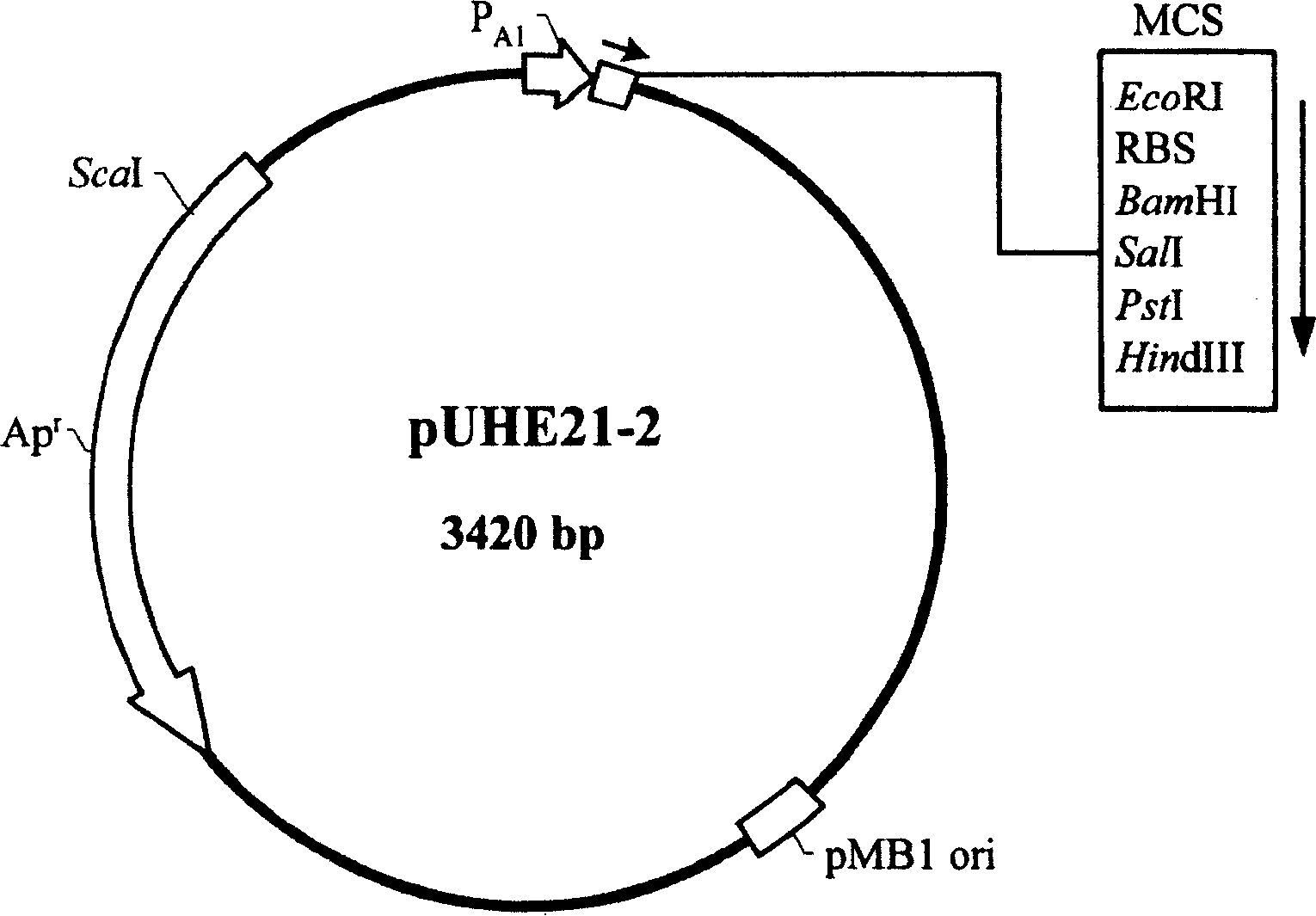
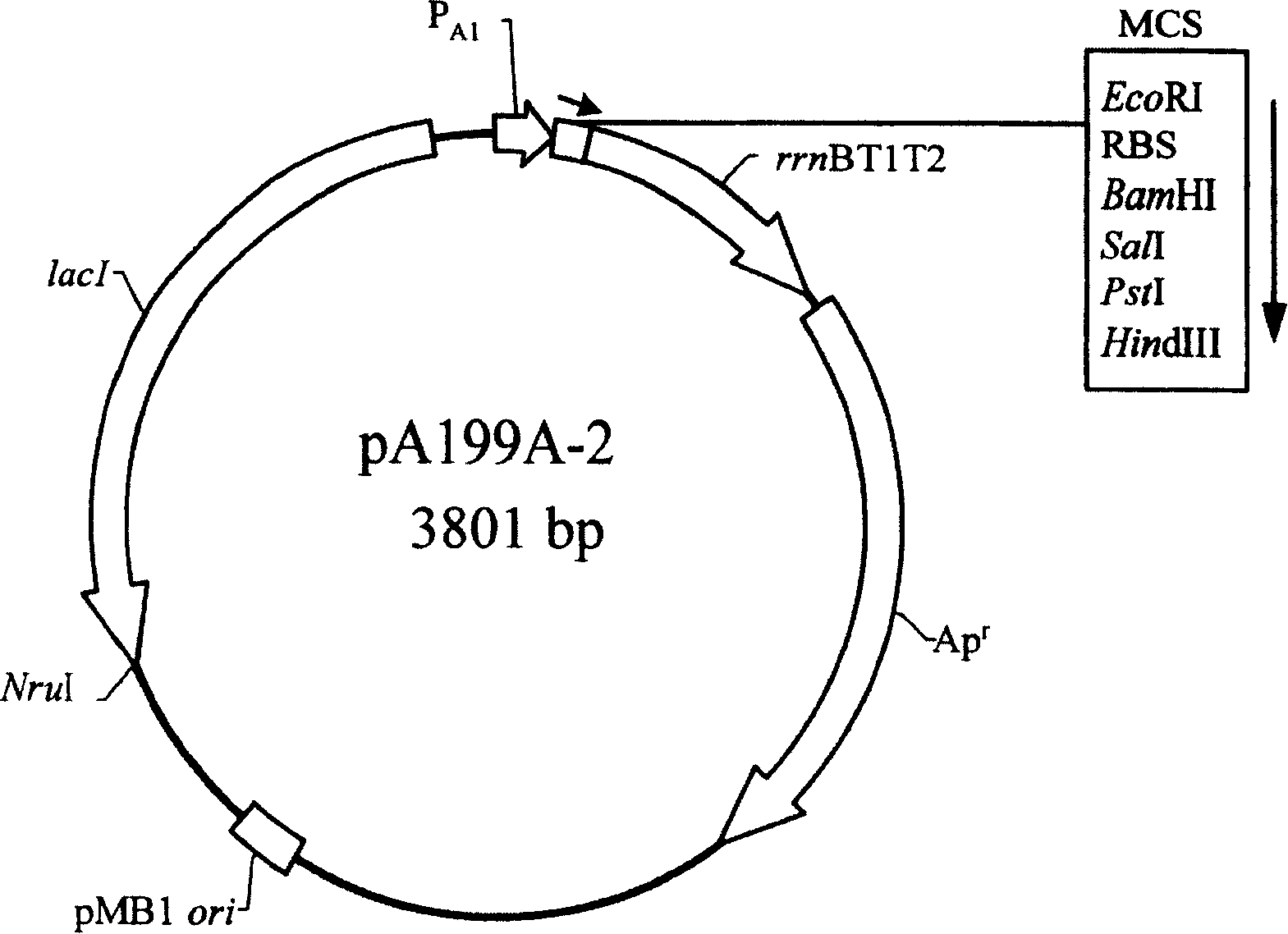
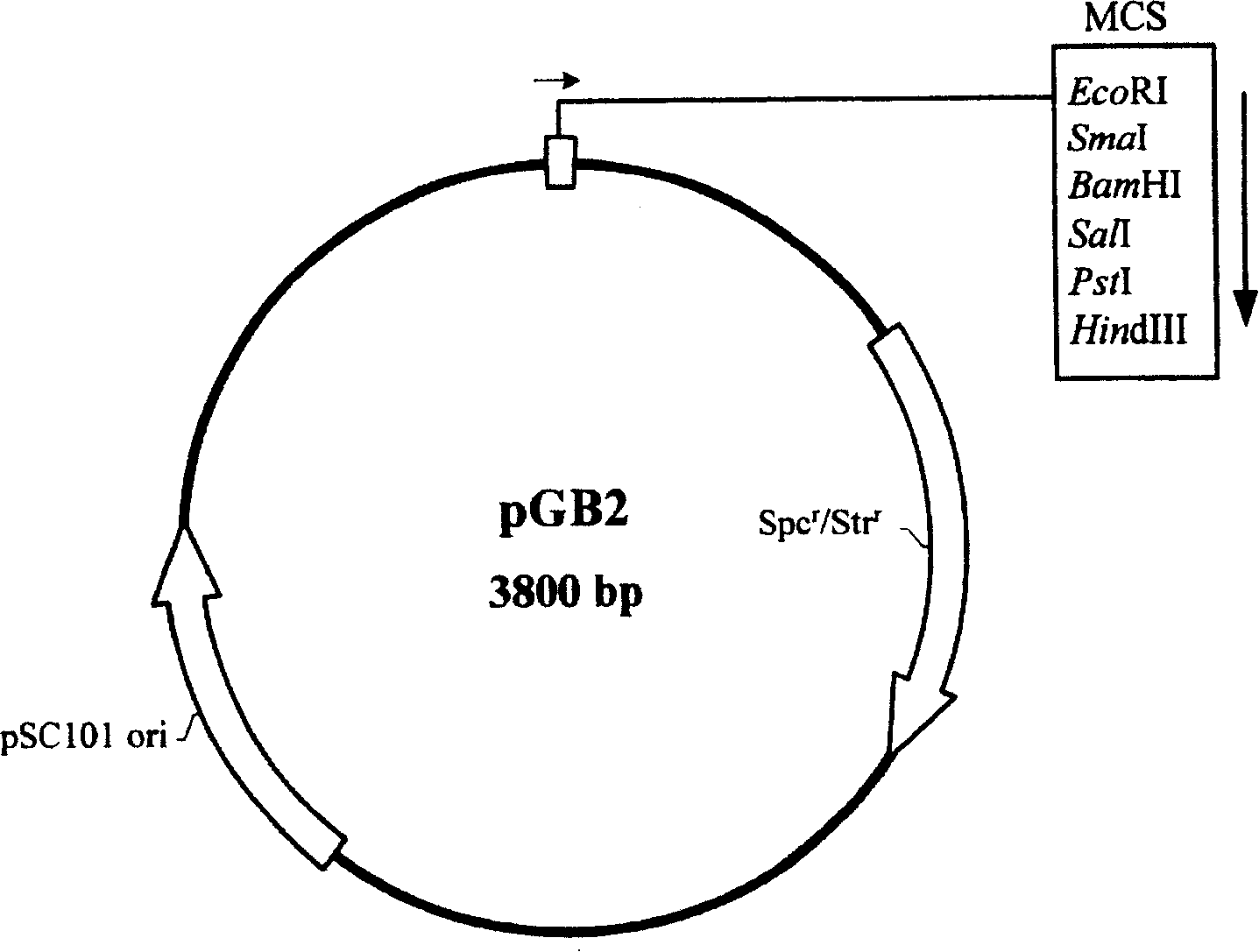
[0110] Example 1. Construction of an expression plasmid comprising the Vitella hyaline hemoglobin structural gene (vgb) and thioredoxin structural gene (trxA)
[0111] Experimental Materials:
[0112] The intermediate cells (intermediate cells) used in the DNA cloning process were Escherichia coli (Escherichia coli) XL1-Blue (Stratagene Co.), and were cultured at 37°C in Luria-Bertani (LB) medium (Miller, J.H. (1972 ), Experiments in Molecular Genetics, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY), then the transformed Escherichia coli was cultured in the medium supplemented with antibiotics (the dosage of antibiotics was 15 μg / mL for streptomycin, and 15 μg / mL for ampicillin Penicillin was used at 50 μg / mL).
[0113] Vitreoscilla sp. (Murray strain no.389) was donated by Dr. Webster (Department of Biology, Illinois Institute of Technology, Chicago, USA), and cultured at 30°C in a medium containing 1.5% yeast Extract (yeast extract), 1.5% peptone (peptone) and 0.02...
Embodiment 2
[0149] Example 2. Production of thioredoxin, Vitreola hemoglobin, and fusion protein of thioredoxin and Vitiligo hemoglobin with Escherichia coli
[0150] The recombinant plasmids pGB-VHb, pGB-Trx, pGB-TV1 and pGB-TV2 obtained in Example 1 were respectively transformed into Escherichia coli strain BL21(DE3) according to the plasmid transformation method described in the "General Experimental Method" above. (Novagen Co.), and obtained recombinant strains BL21(DE3) / pGB-VHb, BL21(DE3) / pGB-Trx, BL21(DE3) / pGB-TV1 and BL21(DE3) / pGB-TV2.
[0151] Colonies of each recombinant strain were selected from the solid-state culture dish, inoculated into 5 mL of LB medium containing 15 μg / mL streptomycin, and cultured overnight at 37°C. Afterwards, the overnight cultured cells were inoculated into a 250 mL flask containing 20 mL of LB medium (containing 15 μg / mL streptomycin), and when the initial cell density reached 0.05 (OD 550 ), culture the newly inoculated bacteria solution in a consta...
Embodiment 3
[0160] Example 3. Production of heterologous protein -- human interferon alpha 2 with transformed Escherichia coli
[0161] The plasmid pET-IFN contains a T 7 promoter to control the expression of the human interferon α2 structural gene, and its construction method is described below.
[0162] The following two primers were synthesized according to the nucleotide sequence of the human interferon α2 structural gene (E.Austruy et al. (1998), Cancer Gene Ther.5:247-256):
[0163] forward primer
[0164] 5'-tgctctcatatgttg atctgcctcaaac-3' (SEQ ID NO: 5)
[0165] reverse primer
[0166] 5'-cgtgctcgag ttaattattccttacttcttaaac-3' (SEQ ID NO: 6)
[0167] The above two primers were designed to have cleavage sites for restriction enzymes NdeI and XhoI (marked underlined), respectively.
[0168] to use The plasmid pIFN2A purified by the Spin Miniprep kit (obtained from Professor Xu Zuan of the National Institutes of Health, which contains the human interferon α2 gene introduced in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com