Application of kuianchun family compound in anti-tumor drug preparing process
An anti-tumor drug, the technology of aminoquinoxaline, which is applied in the fields of chemistry and biology, can solve the problems that quinoxaline compounds have not been reported, and achieve the effect of good tumor killing, strong degradation resistance and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1Y
[0036] The preparation of embodiment 1YZC-J-1
[0037] (1) Preparation of intermediate 2,3-difuryl-6-aminoquinoxaline
[0038] 6.4 g of bifuroyl and 10 g of 1,2,4-benzenetriamine were dissolved in 70 ml of hot ethanol and refluxed for 40 hours. After cooling and filtering, a red solid was obtained. Recrystallized twice from ethanol to obtain 4.2 g of a yellow solid. HPLC: 99%, EMS: 278 (100, M+1).
[0039] (2) Preparation of N, N-dimethylaminoacyl-2,3-difuryl-6-aminoquinoxaline
[0040] Method 1: Dissolve 0.34 g of trimeric phosgene in 30 ml of dichloromethane, 0.6 g of 2,3-difuryl-6-aminoquinoxaline and 0.38 ml of diisopropylethylamine in 160 ml of dichloromethane Slowly added dropwise to the above solution. Stir for half an hour after the addition, dilute 1 ml of dimethylamine into 20 ml of dichloromethane, and quickly add the solution to the above reaction solution. After stirring for 45 minutes, the reaction solution was washed with water three times, dried over anhy...
Embodiment 2Y
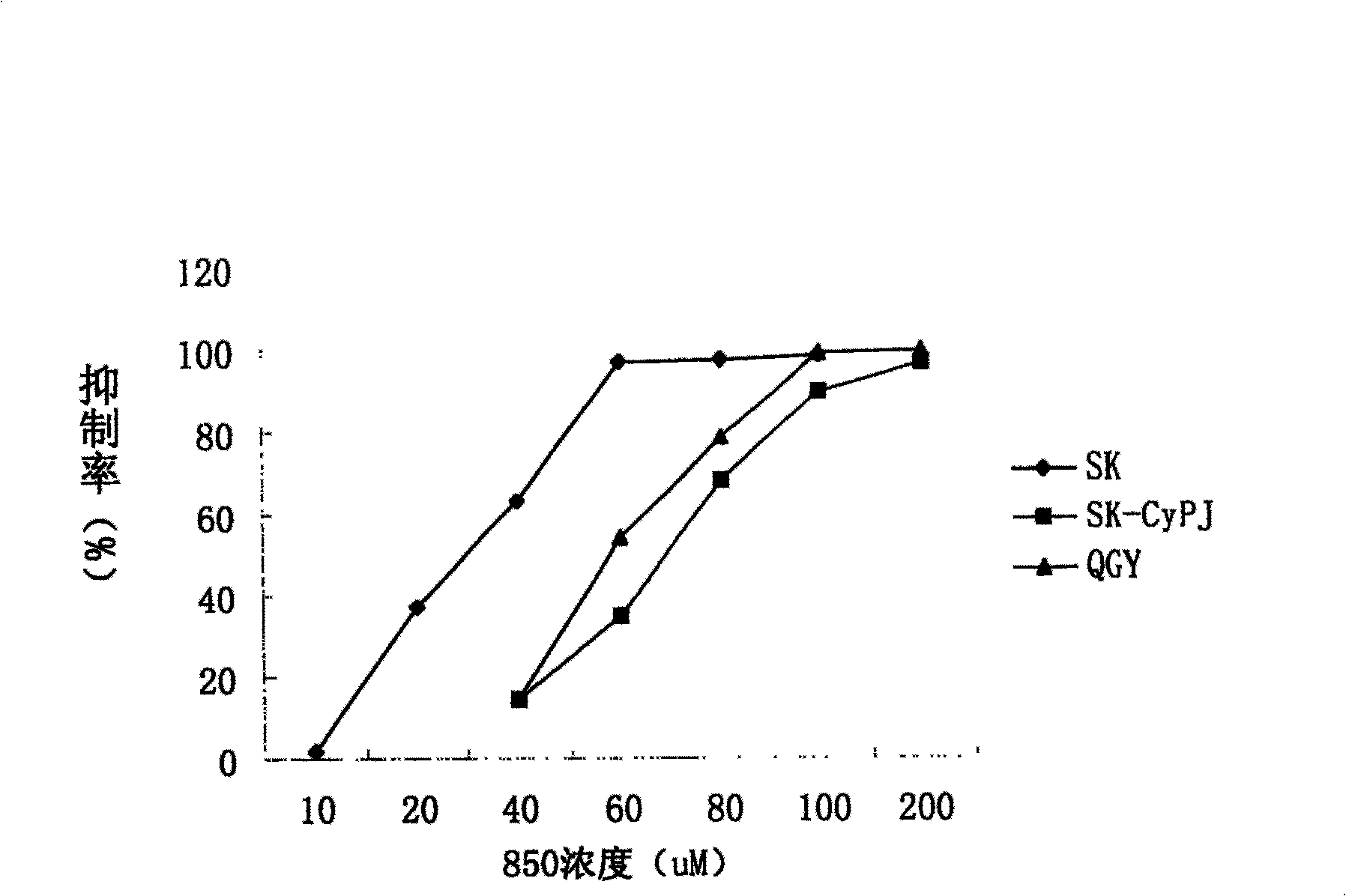
[0052] Inhibitory effect of embodiment 2YZC-J-1 on tumor cell growth
[0053] (1) Cell culture
[0054] Human liver cancer cell lines SK and QGY are derived from the cell lines stored in liquid nitrogen at the Human New Gene Research Laboratory of the Institute of Genetics, Fudan University. The SK-CyPJ transgenic cell line was provided by Dr. Chen Jian of the laboratory. Resuscitate the above-mentioned cells before use: take out the cryopreservation tube from the water bath at 37°C, disinfect it with alcohol, open it, suck out the cell suspension, inject it into the centrifuge tube and add more than 10 times of culture medium (RPMI-1640), mix it at low speed Centrifuge (1000rpm, 5min), remove the supernatant, and wash with culture medium again. After appropriate dilution with culture medium (seeding density was 5×10 5 / ml is appropriate), inoculate the culture bottle, put into CO 2 The incubator was left for static cultivation, and the culture medium was replaced again th...
Embodiment 3
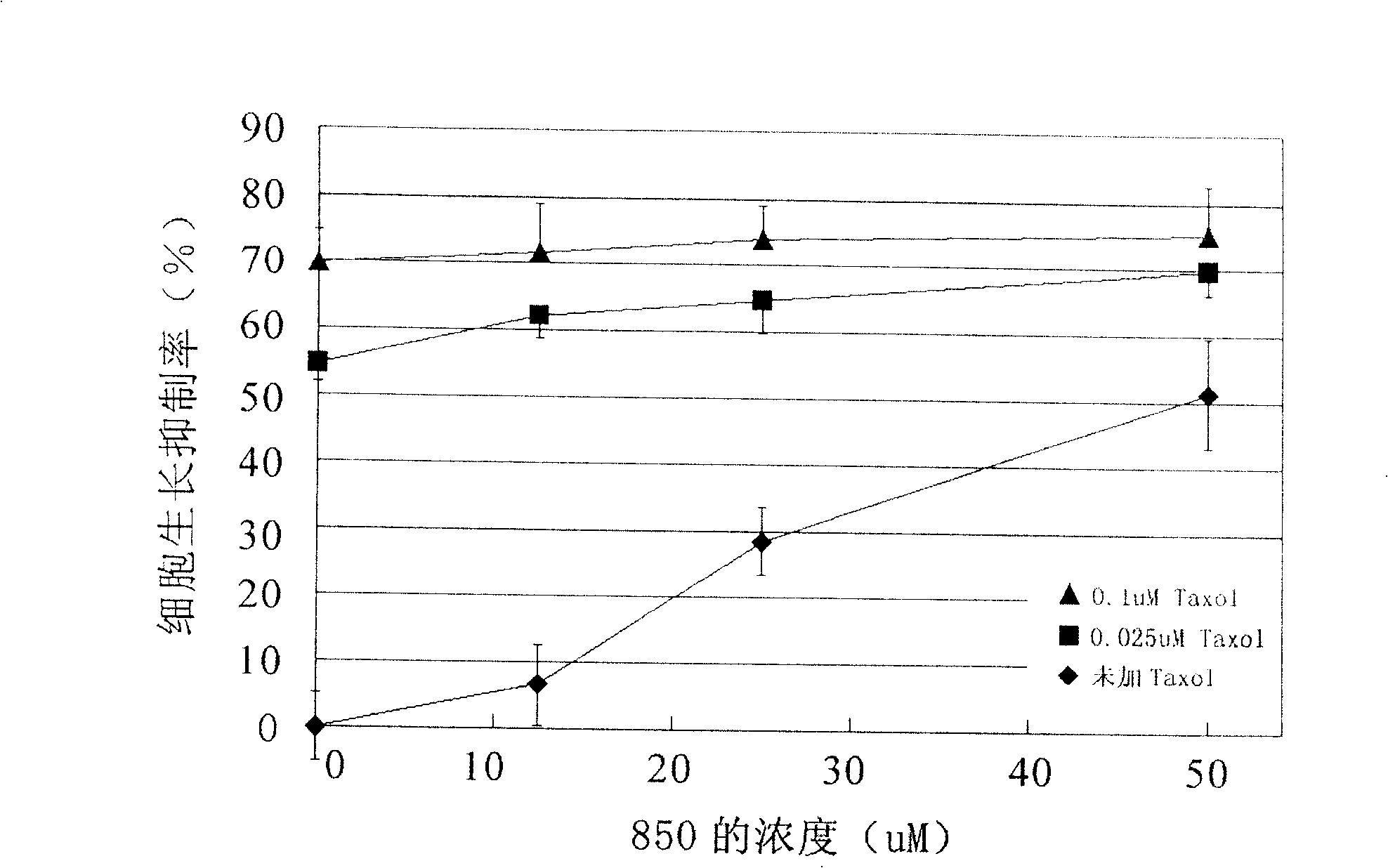
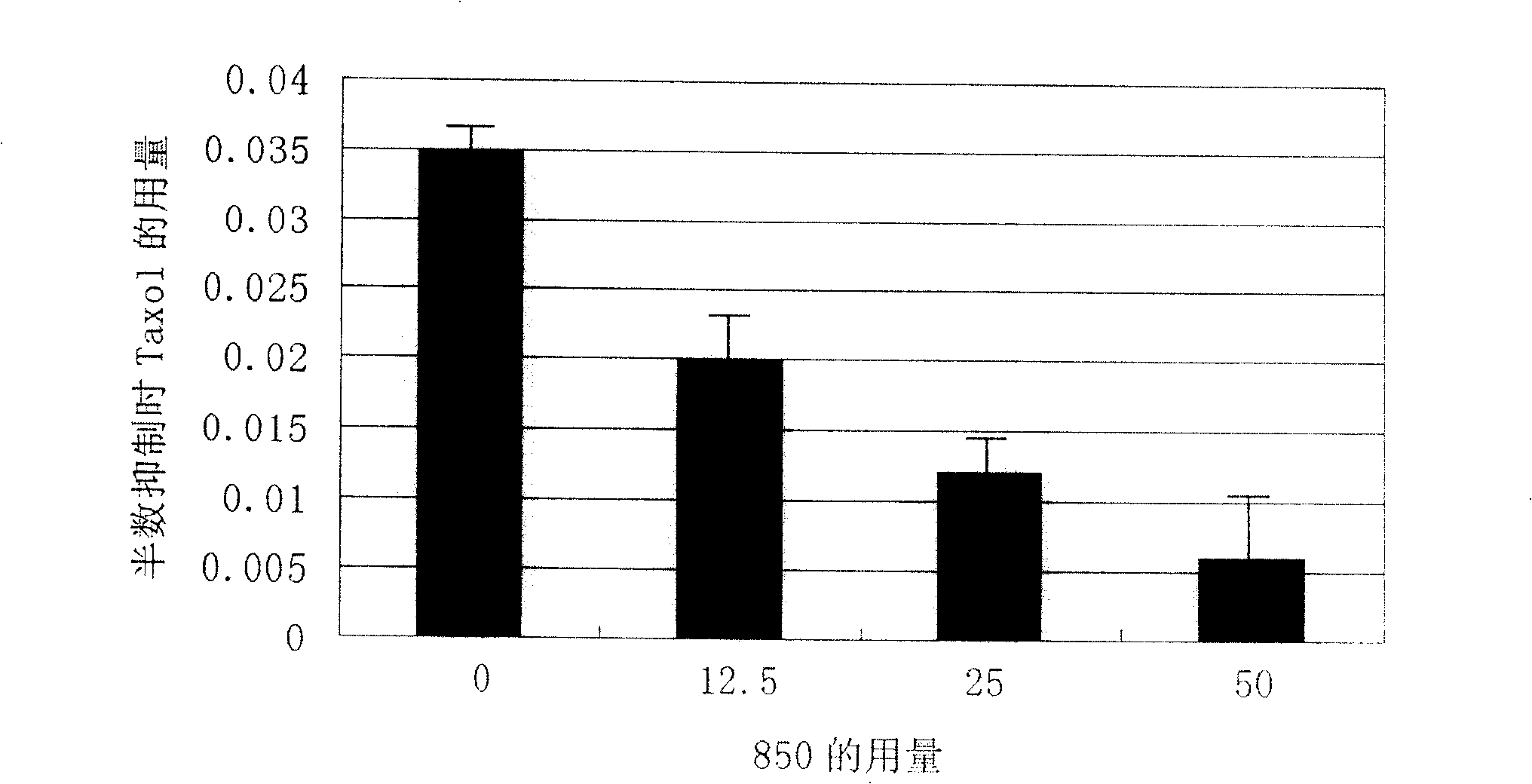
[0066] Embodiment 3 SRB method measures the reducing effect of compound 850 on the amount of taxol
[0067] SK-CypJ cells (2~5*10 3 / well) were inoculated into 96-well plates and cultured for 24 hours to make them adhere to the wall, then added 850 (synthesized by Shanghai Institute of Materia Medica, Chinese Academy of Sciences) and taxol (Shanghai Fushan Biotechnology Co., Ltd.), and determined the different concentrations of 850 and taxol by using the orthogonal method. Inhibition of tumor cell growth. The concentration of 850 is 0uM, 12.5uM and 25uM; the concentration of taxol is 0uM, 0.006uM, 0.025uM and 0.1uM. Set up 6 duplicate wells for each concentration, and set up corresponding zeroing wells and blank controls. Tumor cells at 37°C, 5% CO 2 After culturing under the condition for 72 hours, discard the culture medium, add 100 uL of 10% TCA (Sinopharm Chemical Reagent Co., Ltd.) to each well and fix at 4° C. for 1 hour, discard the fixative, wash 5 times with distil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com