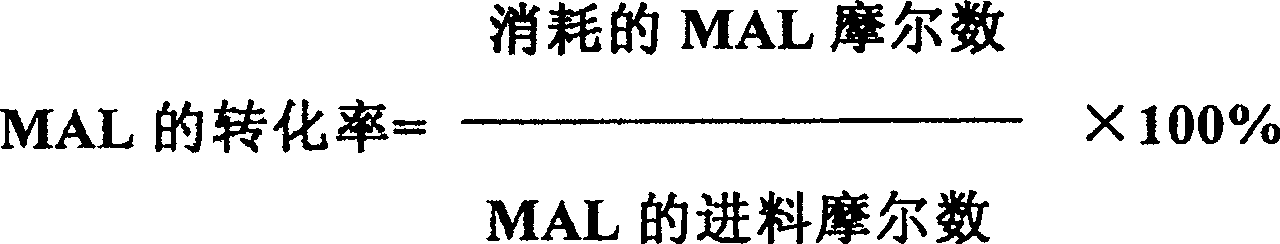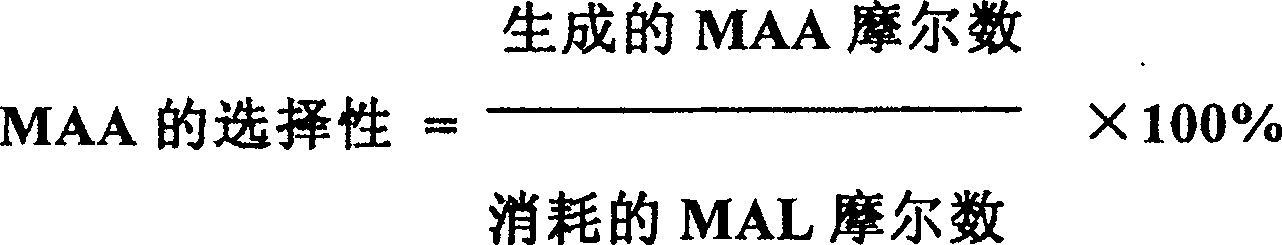Catalyst for selectively oxidizing methyl acrylic aldehyde to synthesize methyl propenoic acid and its use
A technology for synthesizing methacrylic acid and methacrolein, which is applied in the direction of physical/chemical process catalysts, preparation of organic compounds, organic chemistry, etc. It can solve the problems of short service life, reduced catalyst reactivity and selectivity, and reduced mechanical strength of catalysts And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] One with 85 (Mo 12 P 1.5 K 1.5 Sb 0.5 Cu 0.3 As 0.2 V 0.5 In 0.3 Cs 0.2 Mo 2 ) / 15ZrO 2 The preparation method of the one-stage oxide catalyst (constituting the atomic ratio of the metal element of the active component and the carrier heat-conducting diluent) of expression is as follows:
[0017] 105.9 grams of ammonium paramolybdate, 2.9 grams of ammonium metavanadate, 4.2 grams of potassium hydroxide and 2.0 grams of cesium nitrate were dissolved in 200 grams of distilled water to obtain solution A. 7.4 g of phosphoric acid and 1.4 g of arsenic acid were dissolved in 20 g of water to obtain solution B. Dissolve 5.7 grams of antimony trichloride in 30 grams of 10% by weight of dilute hydrochloric acid aqueous solution, then add 3.6 grams of copper nitrate and 4.5 grams of indium nitrate to obtain solution C. Under stirring, solution B was first added to solution A, and then solution C was added, and the pH of the mixed solution was adjusted to 6 with ammonia ...
Embodiment 2
[0020] Except that 2.9 grams of ammonium metavanadate and 4.5 grams of indium nitrate were replaced by 6.5 grams of ammonium paratungstate and 3.2 grams of germanium tetrachloride. All the other catalysts were prepared in the same manner as in Example 1. The prepared catalyst consists of:
[0021] 85 (Mo 12 P 1.5 K 1.5 Sb 0.5 Cu 0.3 As 0.2 W 0.5 Ge 0.3 Cs 0.2 Mo 2 ) / 15ZrO 2
[0022] The selective oxidation reaction was carried out in the same manner as in Example 1, and the result of the reaction at 320° C. (hot spot temperature) for 80 hours was: the MAL conversion rate was 86.2%, and the MAA selectivity was 87.8%.
Embodiment 3
[0024]Except that 2.9 grams of ammonium metavanadate and 4.5 grams of indium nitrate were replaced with 3.3 grams of niobium pentoxide and 2.5 grams of silicon tetrachloride, all the others were prepared in the same manner as in Example 1. The prepared catalyst consists of:
[0025] 85 (Mo 12 P 1.5 K 1.5 Sb 0.5 Cu 0.3 As 0.2 Nb 0.5 Si 0.3 Cs 0.2 Mo 2 ) / 15ZrO 2
[0026] The selective oxidation reaction was carried out in the same manner as in Example 1, and the result of the reaction at 325° C. (hot spot temperature) for 80 hours was: the MAL conversion rate was 85.2%, and the MAA selectivity was 87.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com