Method for the preparation of 2'-deoxy-2',2'-difluoro cytidine
A technology of diflucytidine and nucleosides, applied in the field of stereoselective preparation of 2'-deoxy-2', which can solve the problems of low yield of β-anomer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1: 1-(2'-deoxy-2', 2'-difluoro-5-benzoyl-3-(4-phenyl)benzoyl-D-ribofuranosyl-4-aminopyrimidine- 2-keto
[0095]
Embodiment 1-1
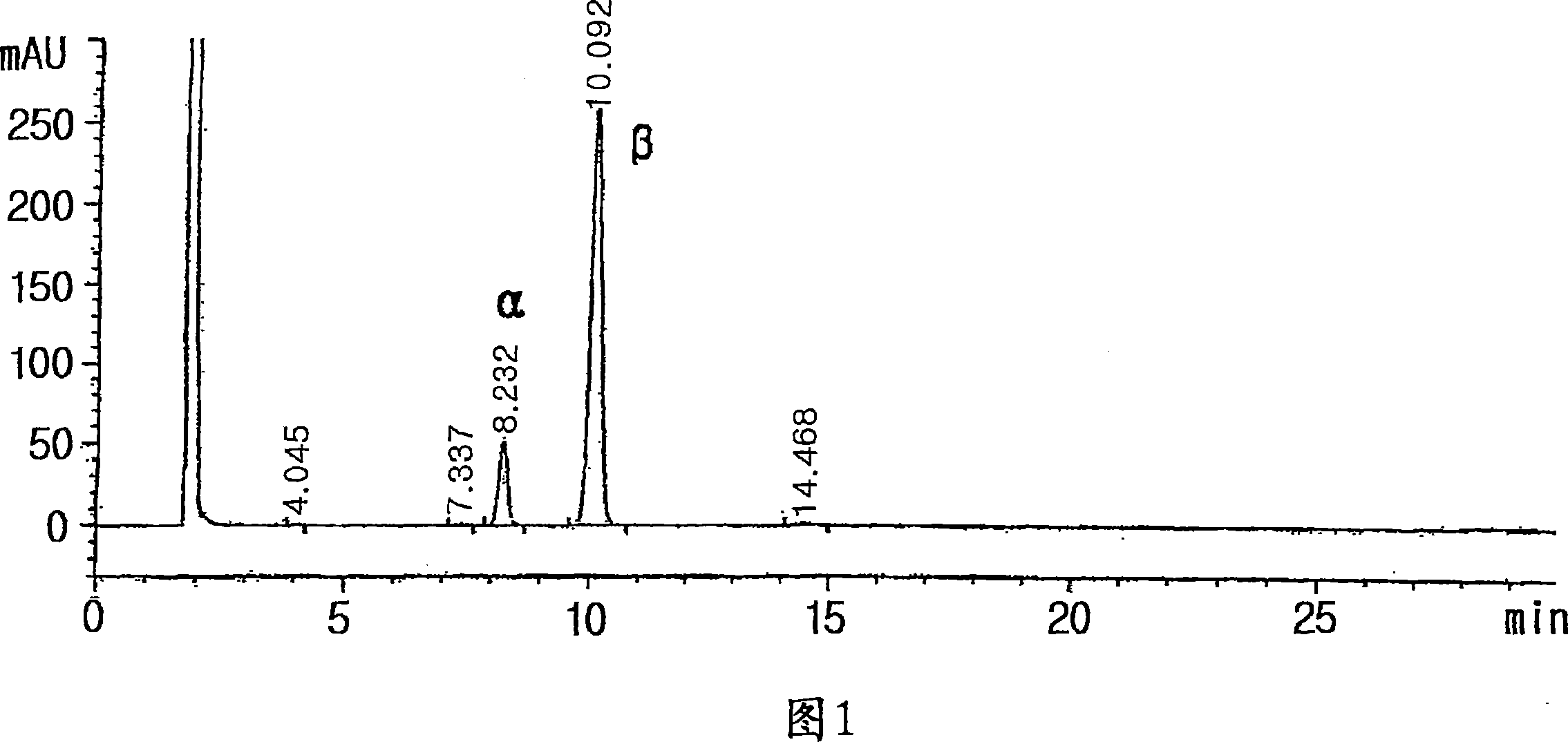
[0097] 44.5g of cytosine, 252ml of hexamethyldisilazane and 252mg of ammonium sulfate were mixed and refluxed until the solution was uniform, and then refluxed for another hour. Thereto was added 200 ml of ethyl acetate and heated to remove remaining unreacted hexamethyldisilazane. To the resulting solution was added a mixture of 160 ml of heptane and 40 ml of diphenyl ether and 10.4 g of 1-α-bromo-2'-deoxy-2',2'-difluoro-D-ribofuranosyl- 5-Benzoyl-3-(4-phenyl)benzoate. The resulting mixture was allowed to react for 8 hours, while diphenyl ether (40ml) / heptane (4l) mixture was added dropwise thereto while distillation was carried out, and the reaction temperature was kept at 130-140°C. This step allows the continuous removal of bromotrimethylsilane from the reaction mixture during the course of the reaction. After the reaction was completed, 140 ml of heptane was added to the reaction mixture. The solution was cooled to 100°C, carefully quenched with 12 ml of water and stir...
Embodiment 1-2
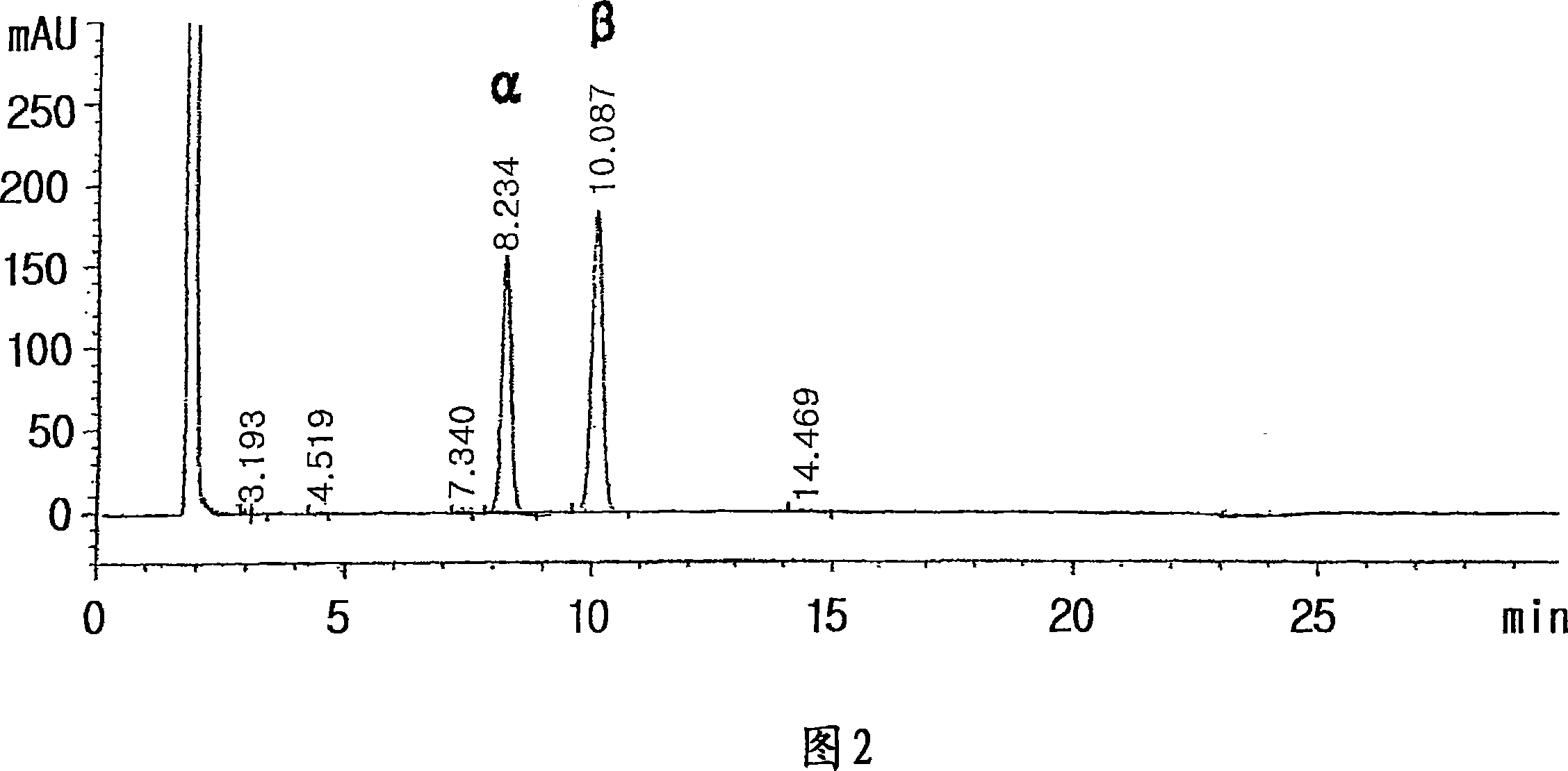
[0101] 11.1 g of cytosine, 63 ml of hexamethyldisilazane and 63 mg of ammonium sulfate were mixed and refluxed for 2 hours. To the resulting mixture was added 60 ml of toluene and heated to remove remaining unreacted hexamethyldisilazane. A mixture of 40 ml of octane and 20 ml of diphenyl ether and 3.5 g of 1-α-bromo-2'-deoxy-2',2'-difluoro-D-ribofuranosyl-5 obtained in Preparation 1 were added to the resulting solution - Benzoyl-3-(4-phenyl)benzoate. The resulting mixture was allowed to react for 10 hours, while diphenyl ether (10 ml) / heptane (1 l) mixture was added dropwise thereto while distillation was performed, and the reaction temperature was maintained at 140-150°C. This step allows the continuous removal of bromotrimethylsilane from the reaction mixture during the course of the reaction. After the reaction was completed, 50 ml of heptane was added to the reaction mixture. The solution was cooled to 80-100°C, 12ml of water was carefully added dropwise, and the mixtu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com