Process for isolation of crystalline tacrolimus
A technology of tacrolimus and crystallization, applied in the field of separation and crystallization of tacrolimus, to achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Isolation of tacrolimus from whole fermentation broth
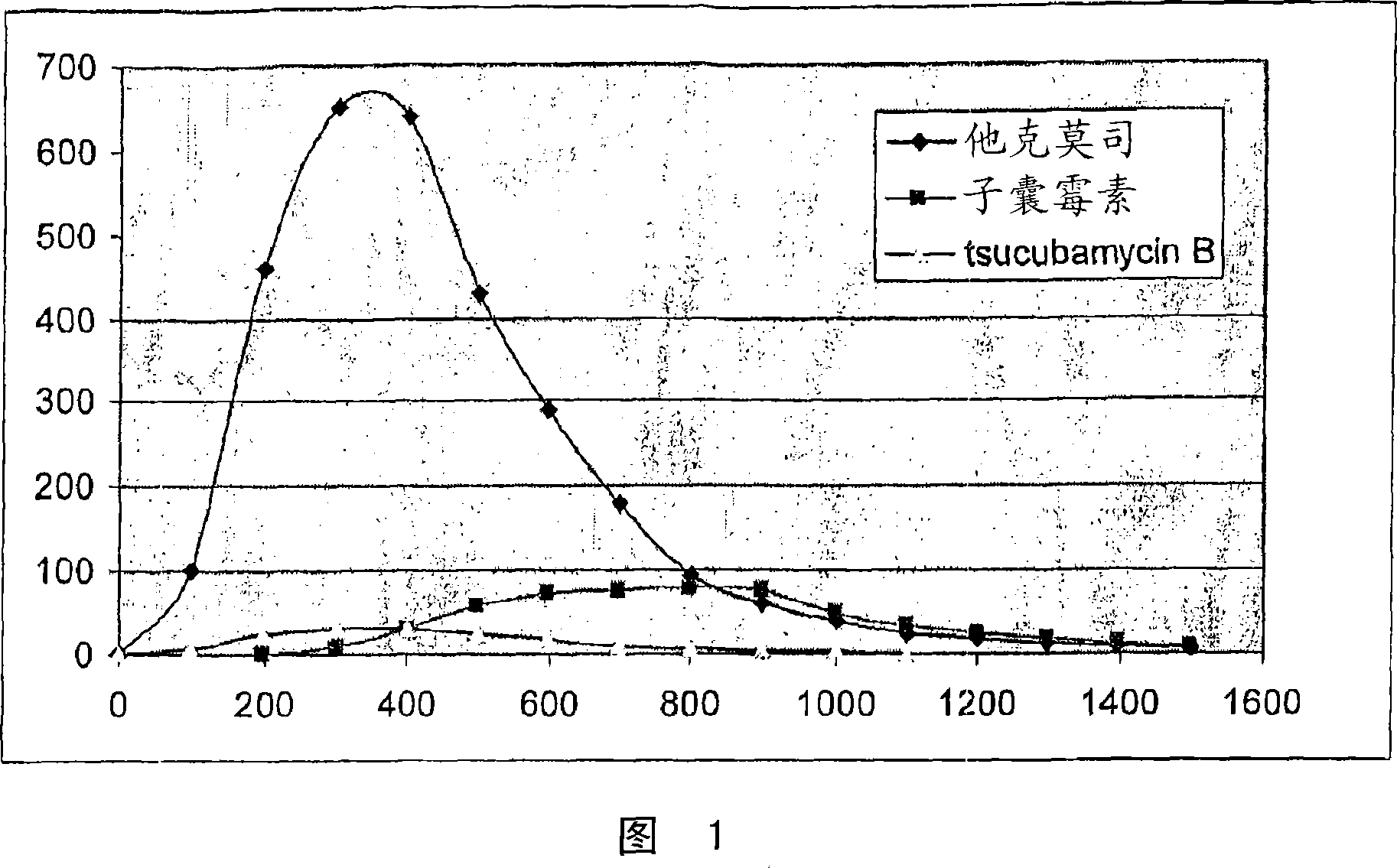
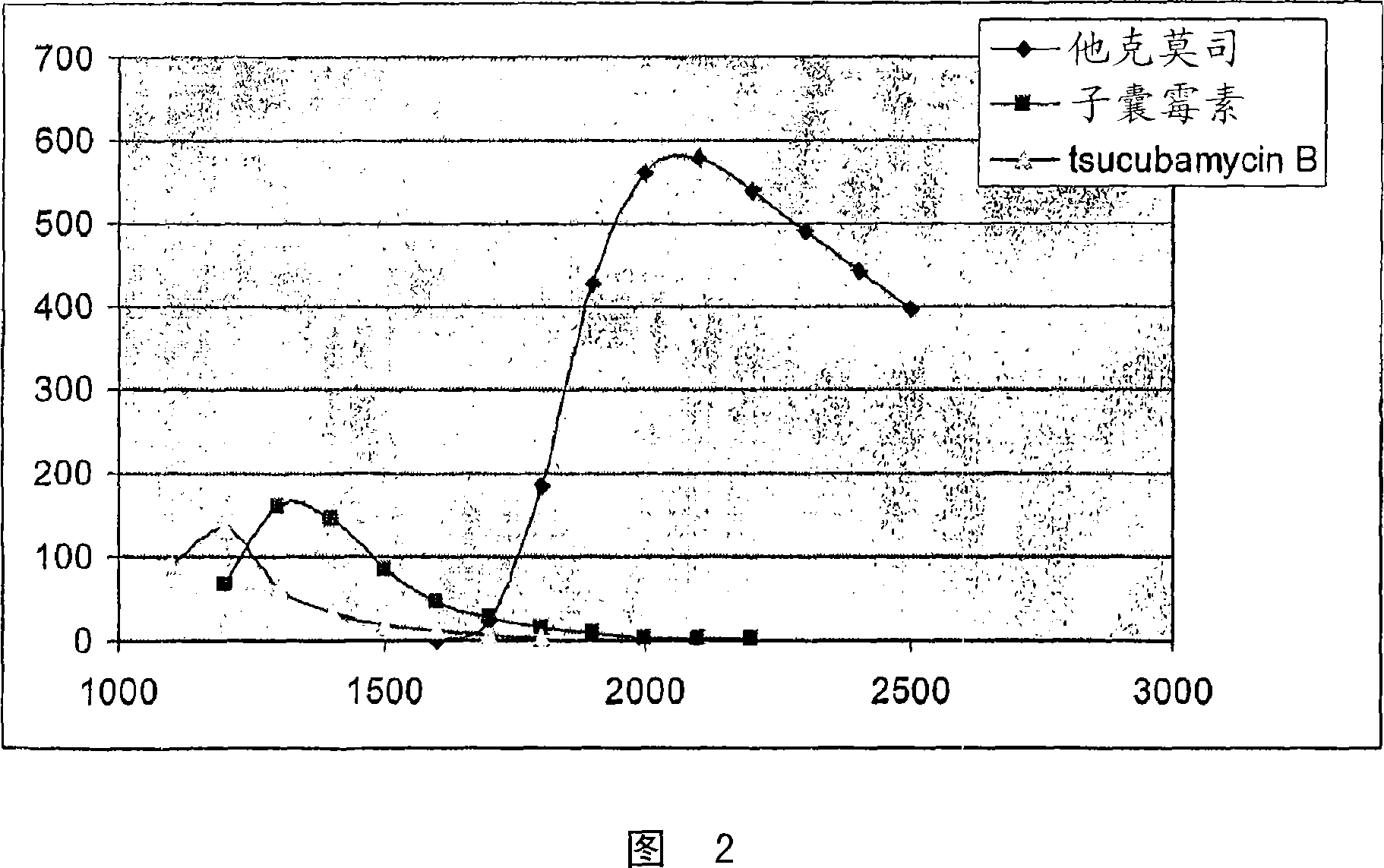
[0033] 10.0 liters of the total fermentation broth obtained by submerged culture of tacrolimus-producing Streptomyces sp. was diluted with 10.0 liters of 2-propanol and the suspension was stirred for 4 hours. The solid phase was separated by filtration and the filtrate was extracted twice with 1000 ml toluene. The combined toluene extracts were evaporated under reduced pressure to a volume of approximately 25 ml and according to HPLC analysis, the concentrate contained 2.12 g tacrolimus, 0.25 g ascomycin and 0.11 g tsucubamycin B. The concentrate was loaded onto a 20 g silver nitrate modified chromatography column packed with 200 g silica gel (Lichroprep Merck 60, 25-40 μm). The column was first washed with toluene (approximately 400 ml) and then with toluene progressively polarized with isobutyl methyl ketone (up to 60% (v / v)). The pure tacrolimus-containing fractions (HPLC monitoring) were combine...
Embodiment 2
[0035] Isolation of tacrolimus from dried mycelium
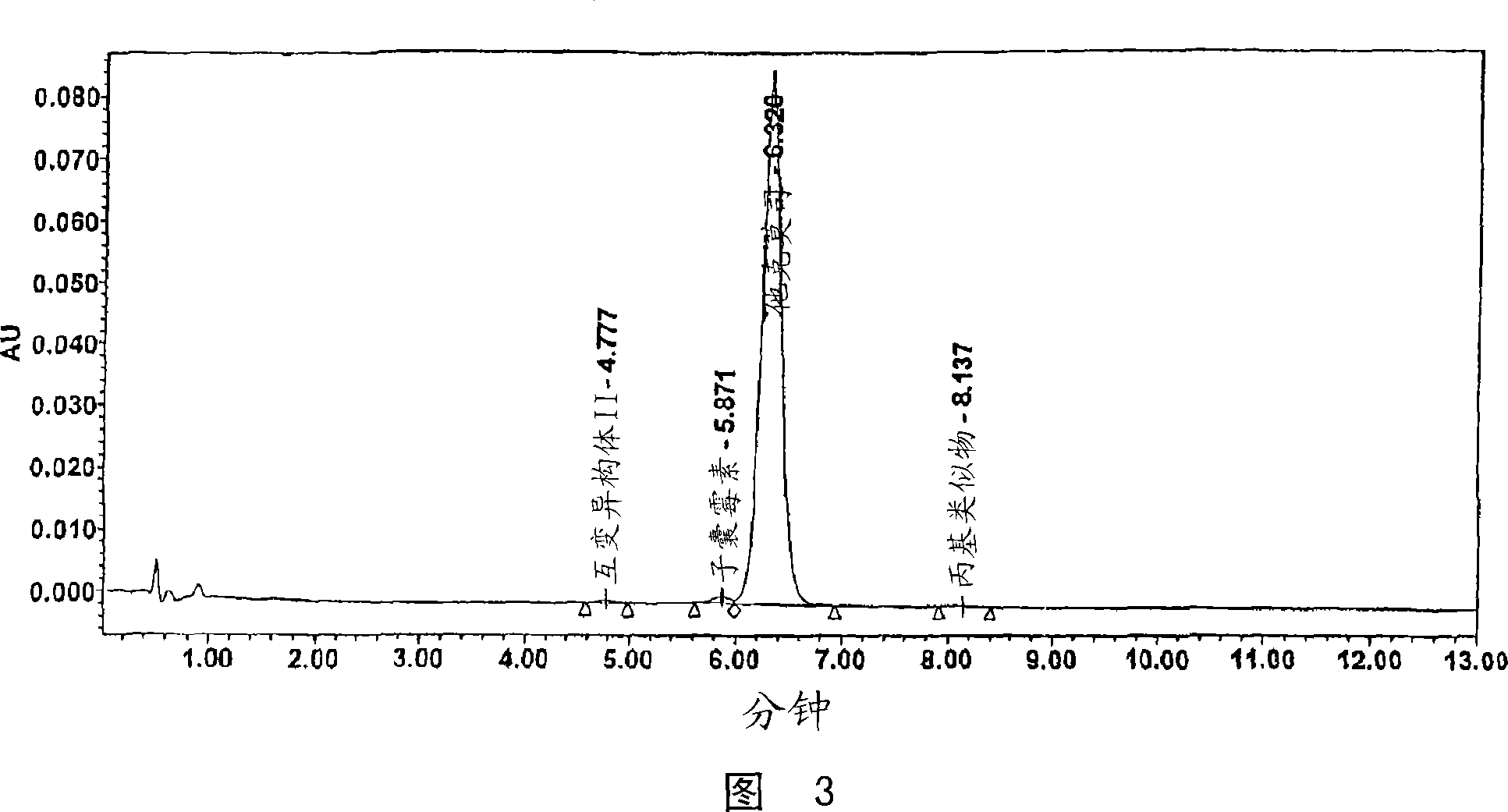
[0036] 40.0 kg of dry mycelium containing 0.21% tacrolimus according to HPLC analysis was prepared by processing 200 liters of fermentation broth obtained from submerged culture of tacrolimus-producing Streptomyces sp. The mycelium was extracted with 50% (v / v) acetone to obtain 40.0 liters of an aqueous extract, which was then extracted twice with 4 liters of toluene to obtain 15 liters of an organic extract. The organic extract was concentrated to a volume of about 1 liter. The concentrate was packed into a column containing 4.0 kg of silica gel (Merck 100, 63-200 μm). The column was washed first with toluene (approximately 30 liters), then with propanol progressively polarized toluene (up to 20% (v / v) acetone). Fractions containing tacrolimus (monitored by TLC) were combined and evaporated to dryness to give a residue (residue after first chromatography, 130 g) containing 61.6% tacrolimus, 7.9% Ascomycin and...
Embodiment 3
[0038] Preparation of silver nitrate modified silica gel
[0039] 10.0 g of crystalline silver nitrate were dissolved in 1000 ml of methanol under heating and 100 g of silica gel (Lichroprep Merck 60, 25-40 μm) were added to the solution, then the suspension was evaporated to dryness and the residue was dried at 60 mbar vacuum and 70 °C .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com