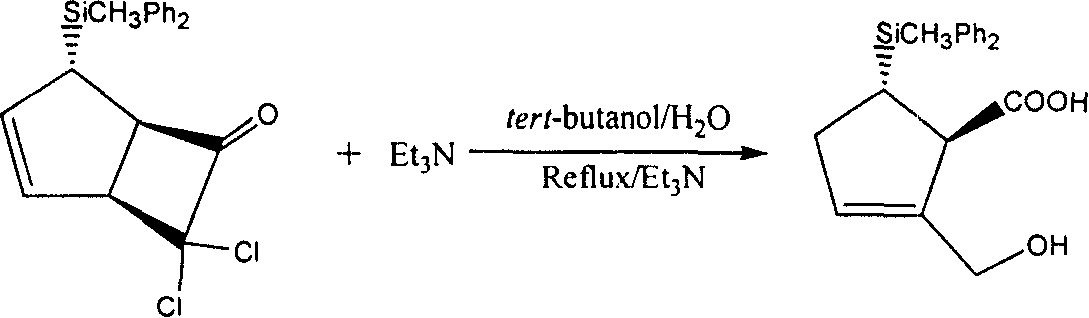Method for synthesizing medication Entecavir of anti hepatitis B
A technology of entecavir and formula, applied in the synthesis field of anti-hepatitis B drugs, can solve the problems of low purity and high cost of entecavir, and achieve the effects of high product purity, low price and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
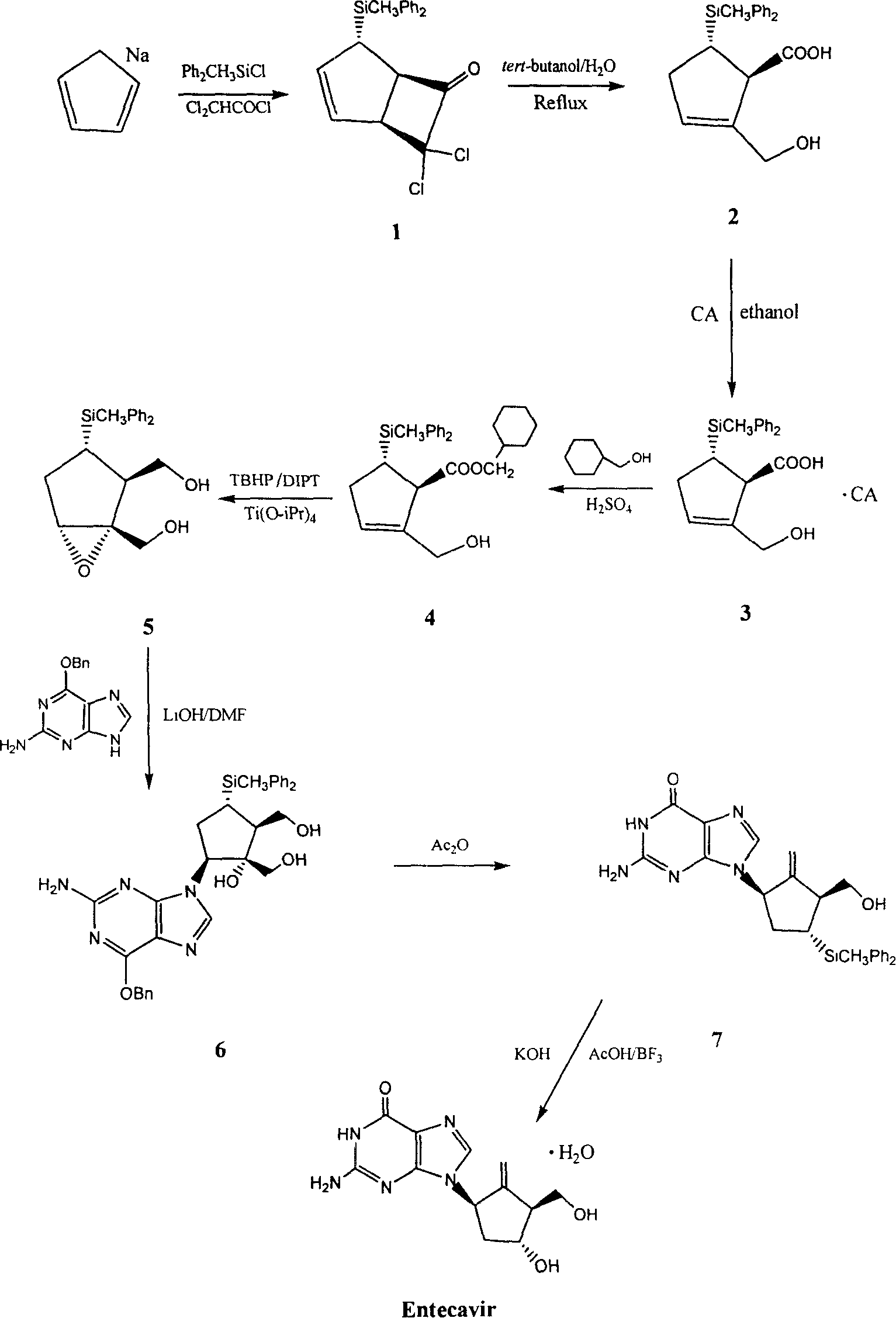
[0041] Preparation of Entecavir
[0042]
[0043] Each specific process in the above-mentioned reaction is respectively:
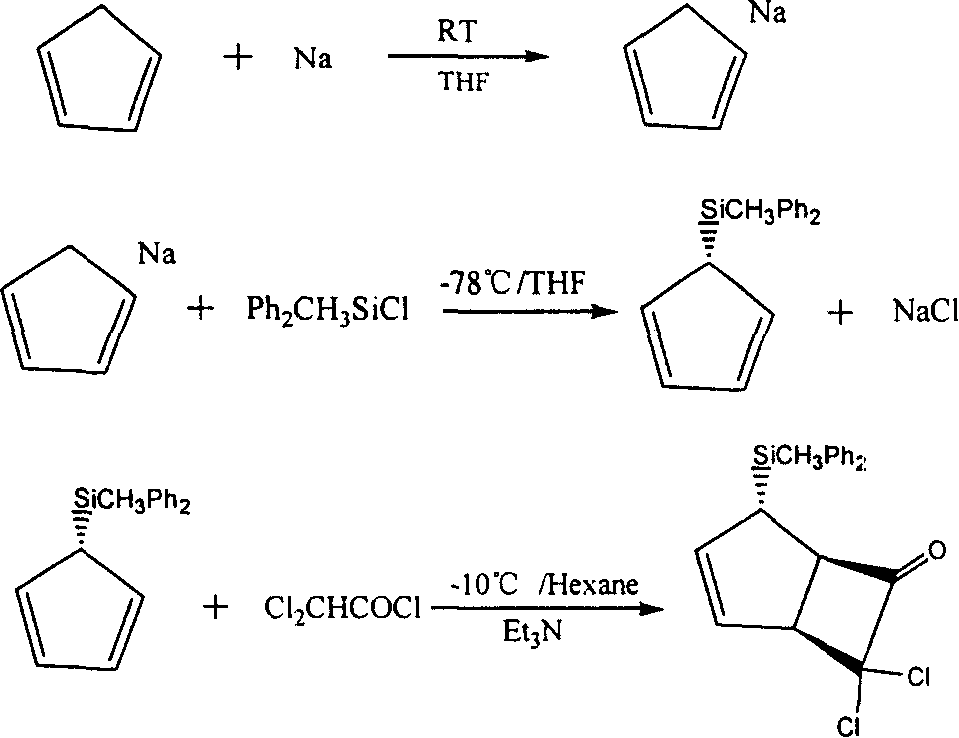
[0044] 1. Preparation of (1α, 4α, 5α)-7,7-dichloro-4-(diphenylmethylsilyl)-cyclo(3,2,0)-heptane-2-cyclo-6-one
[0045] a) Reaction equation
[0046]
[0047] b) Detailed operation process
[0048] 100g of dicyclopentadiene was added to a 250ml four-necked flask, N 2 Under protection, the temperature was heated to 180-190°C, and then the fraction at 40-42°C was slowly collected to obtain 65 g of cyclopentadiene monomer.
[0049] In a 500ml four-necked bottle equipped with mechanical stirring, add 300ml of THF and 20g of sodium metal, 2 65 g of cyclopentadiene monomer was added dropwise under protection, and then stirred and reacted at room temperature for 10 hours. After the reaction, the unreacted solid was filtered off and used directly for the next reaction.
[0050] In another 2L four-neck flask equipped with mechanical stirring, with a 500m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com