Modulation of ovulation
A technology of ovulation rate and structural domain, applied in the direction of peptide/protein composition, drug combination, peptide, etc., can solve the problem of not being associated with the effect of regulating ovulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
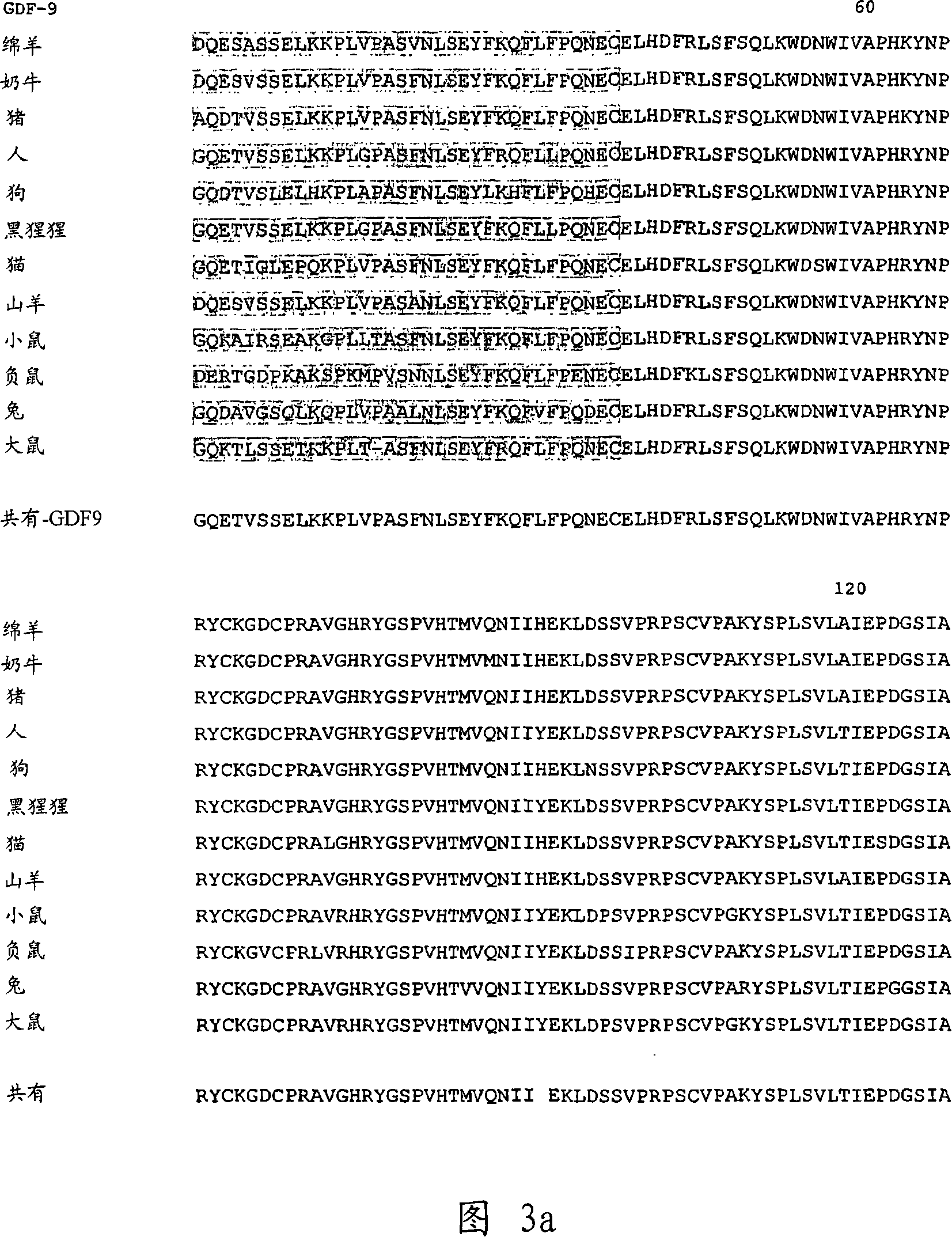
[0094] Use of peptides and antibodies to the N-terminal domains of GDF-9 and GDF-9B in regulating ovulation in sheep.
[0095] Four 12-15mer peptides were synthesized that were identical to the N-terminal domains of the GDF-9 and GDF-9B protein sequences and included additional residues to facilitate their conjugation to keyhole limpet hemocyanin (KLH ) to produce antigen. The peptide sequence is:
[0096] DQESASSELKKPLV(C) (SEQ ID NO: 3);
[0097] SEYFKQFLFPQNEC (SEQ ID NO: 4);
[0098] QAGSIASEVPGPSR(C) (SEQ ID NO: 5); and
[0099] SREHDGPESNQC (SEQ ID NO: 6);
[0100] In this study, groups of 10 nonestrous Romney ewes were injected with each peptide-KLH conjugated antigen dissolved in Freund's complete adjuvant at a dose of 0.4 mg / ewe, and 10 ewes were Nonestrous Romney ewes were injected with KLH antigen at a dose of 0.4mg / ewe as a control group. Animals were then boosted (subcutaneously) at 4 time points at monthly intervals with additional antigen (0.2 mg / ewe at ea...
Embodiment 2
[0112] Regulation of bovine ovulation by peptides and antibodies of the GDF-9 and GDF-9B N-terminal domains.
[0113] Two 15mer peptides were synthesized that were identical to the N-terminal domains of the bovine GDF-9 and GDF-9B protein sequences and included additional residues where required to facilitate their conjugation to KLH for antigen production. The peptide sequence is:
[0114] DQESVSSELKKPLV (C) (SEQ ID NO: 7); and
[0115] QAGSIASEVPGPSR (C) (SEQ ID NO: 5).
[0116] Note that SEQ ID NO:7 is a functional variant of SEQ ID NO:3 comprising a single amino acid change (A→V at amino acid position 5).
[0117]In this study, groups of 10 Friesian cross heifers were injected with each peptide-KLH conjugated antigen dissolved in 2 ml of Freund's complete adjuvant at a dose of 0.4 mg / heifer for 4 days. Injection of 0.5 ml each time; 10 heifers were injected with peptide-KLH conjugated antigen at 0.4 mg / heifer, and 10 heifers were injected with KLH antigen as a control g...
Embodiment 3
[0141] Antibody pairs raised against GDF-9 and GDF-9B peptide fragments 3 Effect of H thymidine incorporation into rat granulosa cells in vitro.
[0142] Using the method described previously (McNatty et al., 2005), various antibody preparations were tested for their ability to neutralize the effects of sheep or murine GDF-9 and sheep GDF-9B on rat granulosa cells when added directly to granulosa cell cultures. A total of 100 μg / ml IgG was added to each treatment and consisted of IgG purified from sheep immunized with GDF-9 or GDF-9B peptide fragments, compared with IgG purified from sheep immunized with KLH. Antibodies are able to neutralize the effects of the growth factors they are directed against (see Figures 5 and 6). It was also shown that the response was dose-dependent in the 2 tested antibody samples against sheep GDF-9 and GDF-9B (P<0.001, see Figures 7 and 8).
[0143] Sheep and mouse GDF-9 and sheep GDF-9B against peptide fragments of the N-terminal region of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com