Pharmaceutical composition containing hardly water soluble medicament
A pharmaceutical composition, water-insoluble technology, applied in the direction of drug combination, medical preparations containing active ingredients, medical preparations without active ingredients, etc., can solve problems such as inability to preserve for a long time, insufficient effect, crystallization, etc. To achieve the effect of ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
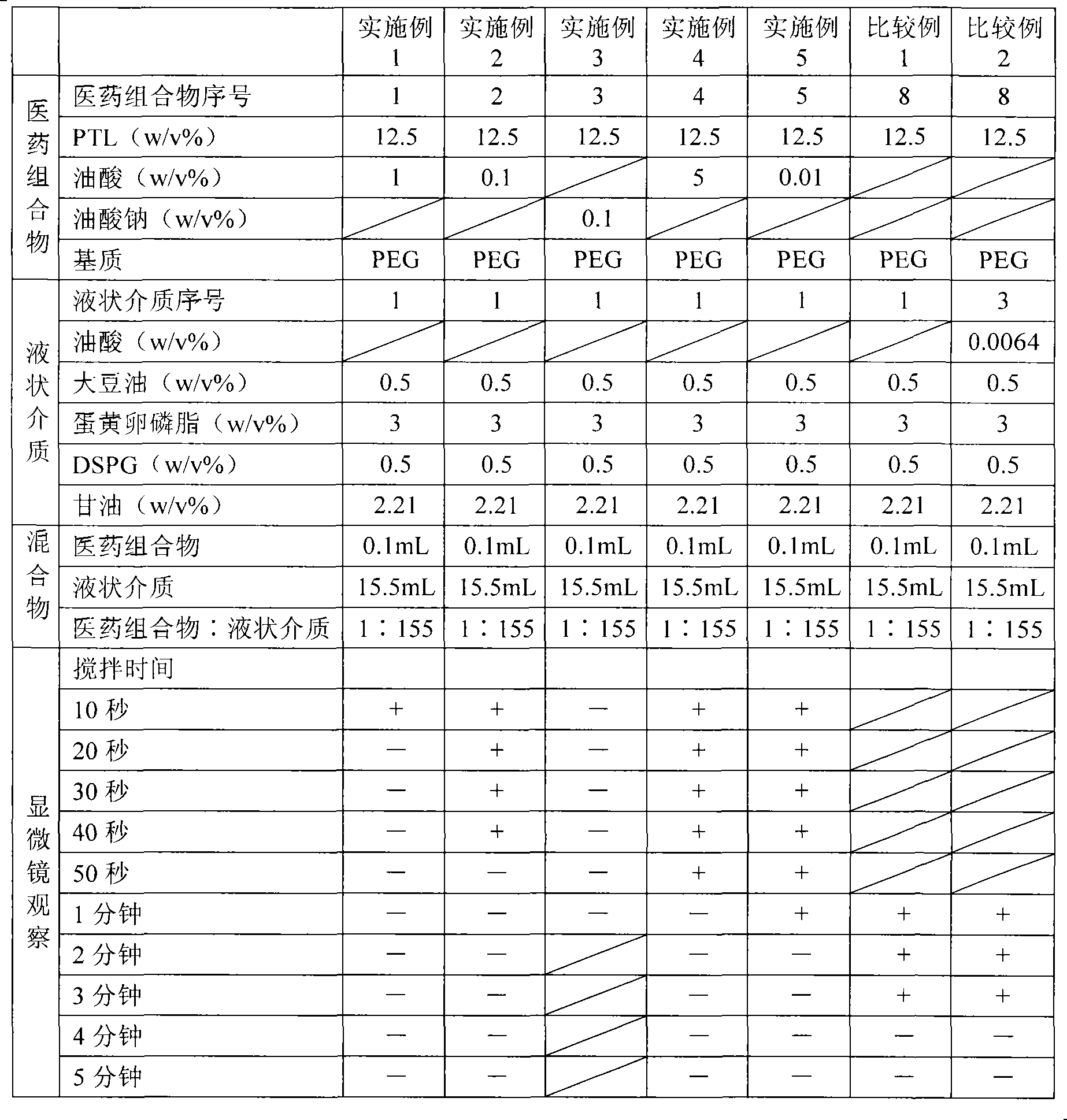
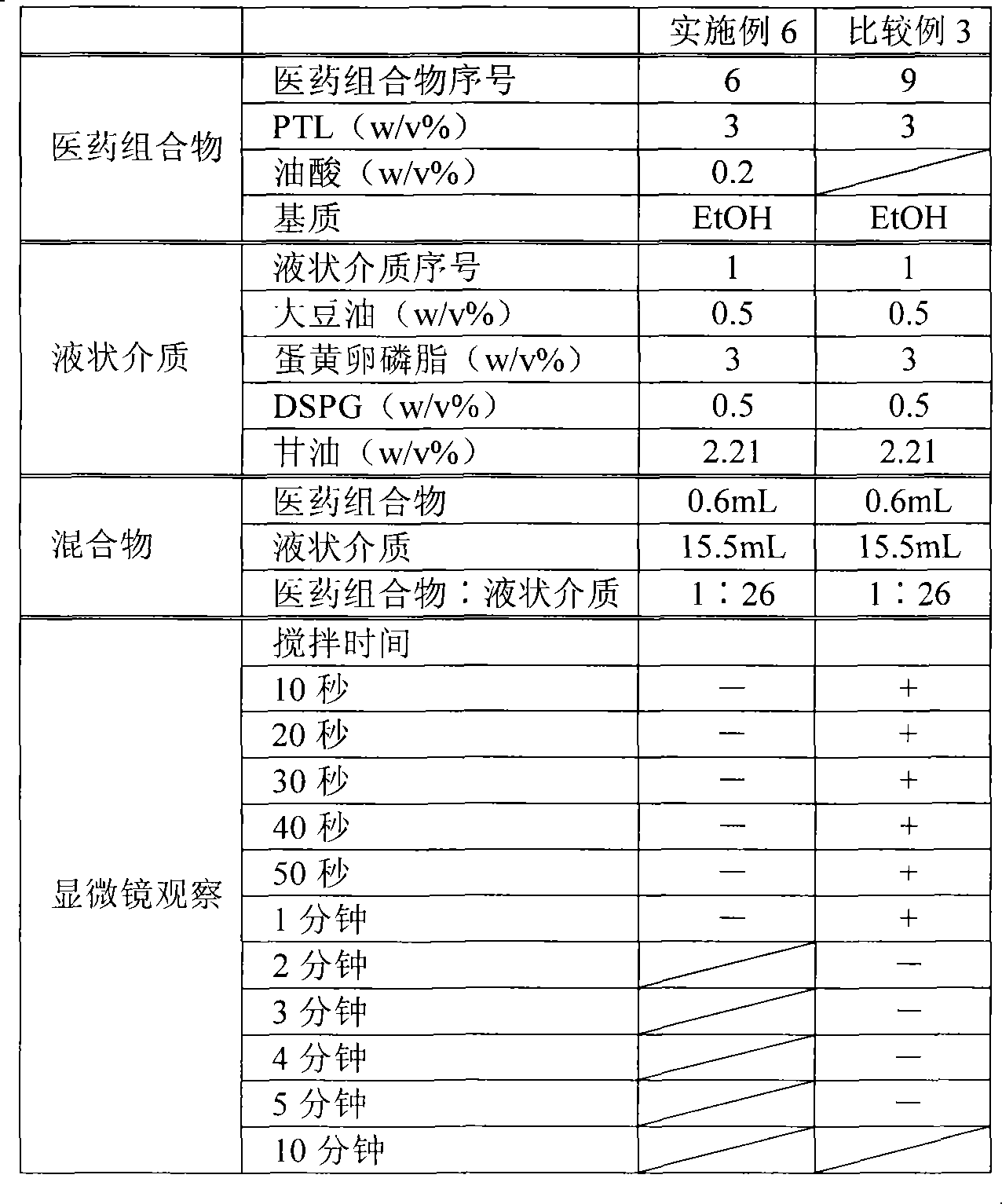
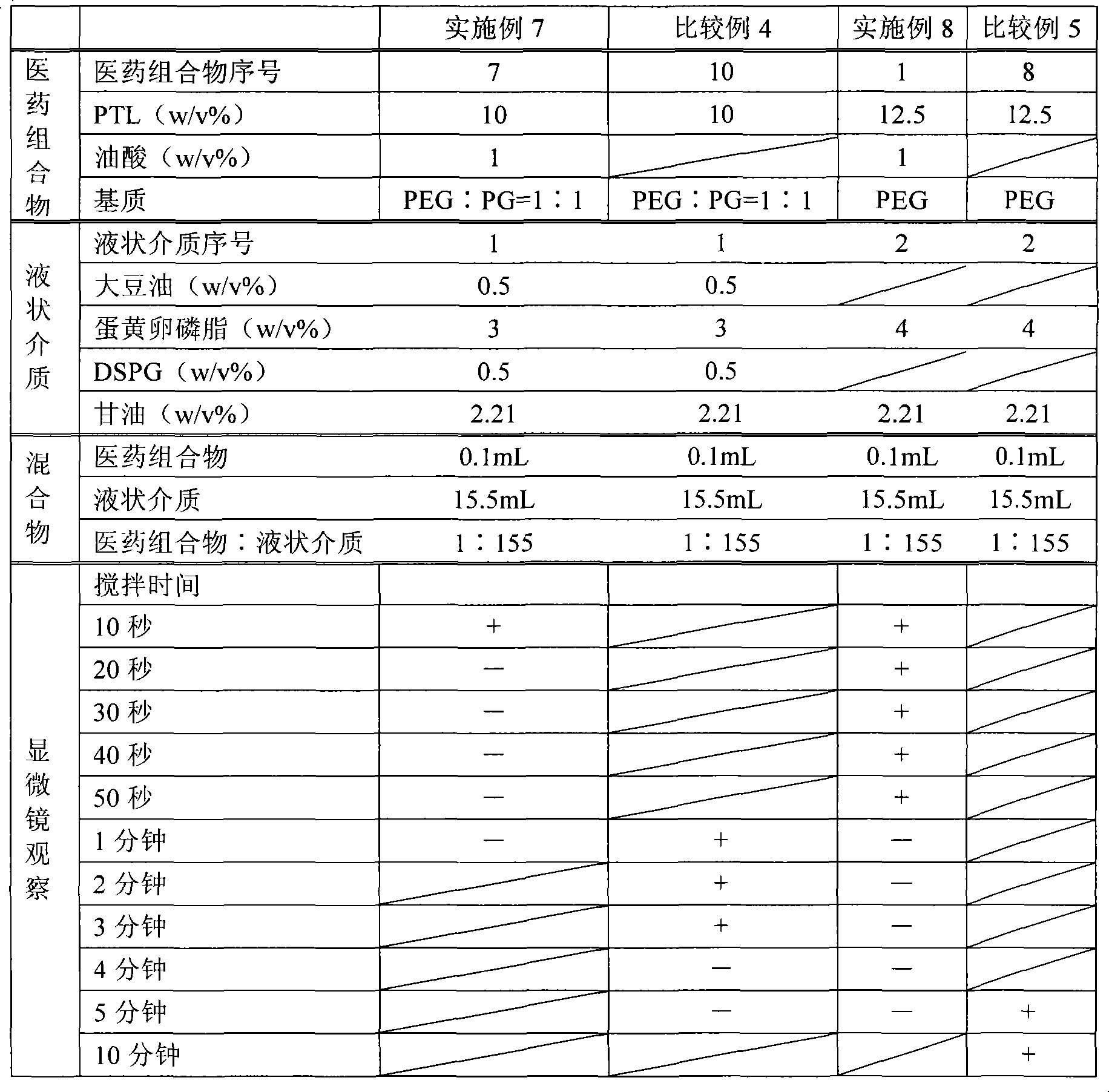
[0089] The components shown in Table 1 below were mixed and dissolved, filtered through a filter with a pore size of 0.2 μm (manufactured by Pall Corporation) made of a polyethersulfone membrane, filled with 5 mL in a glass bottle with a capacity of 5 mL, sealed, and prepared as Pharmaceutical composition 1.
[0090] Table 1 Pharmaceutical composition 1
[0091] Element
Weight per 100mL
PTL
12.5g
Oleic acid
1g
PEG
Appropriate amount
[0092] After mixing the ingredients shown in Table 2 except glycerin, add glycerin dissolved in water for injection to make the final concentration of glycerol reach 2.21w / v%, and use a Polytron homogenizer (manufactured by KINEMATICA Co., Ltd. ), coarsely emulsified at 25000rpm for 10 minutes under nitrogen flow and heating.
[0093] Then, the obtained coarse emulsion was subjected to a high-pressure homogenizer (manufactured by APV Co., Ltd.), under a nitrogen flow at an emulsifica...
Embodiment 2
[0097] The components shown in the following Table 3 were mixed and dissolved, filtered, filled and sealed in the same way as the pharmaceutical composition 1 in preparation example 1, and the pharmaceutical composition 2 was prepared. As the liquid medium, the same liquid medium (liquid medium 1) as in Example 1 was prepared and used.
[0098] Element
Embodiment 3
[0100] The components shown in Table 4 below were mixed and dissolved, filtered, filled and sealed in the same way as the pharmaceutical composition 1 in preparation example 1, and pharmaceutical composition 3 was prepared. As the liquid medium, the same liquid medium (liquid medium 1) as in Example 1 was prepared and used.
[0101] Table 4 Pharmaceutical composition 3
[0102] Element
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com