Reactive orchil component and uses thereof
A reactive dye and composition technology, applied in the field of red dye composition for discharge dyeing, to achieve the effect of excellent dyeing depth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
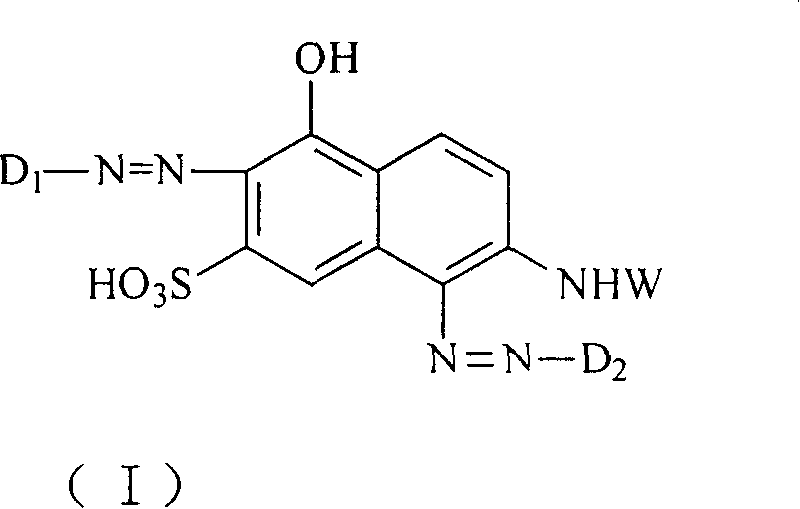
[0060] Take 36.1 parts of 1-aminobenzene-2-sulfonic acid-4-β-sulfonic acid (1-aminobenzene-2-sulfonicacid-4-β-sulfatoethylsulfone) dissolved in 1000 parts of ice water, add 24 parts 32% hydrochloric acid (HCl) aqueous solution was fully stirred, then 7.0 parts of sodium nitrite (sodium nitrite) aqueous solution was added, and the stirring was continued at a temperature of 0-5° C. until the diazotization was completed. Then, 23.9 parts of 2-amino-5-hydroxynaphthalene-7-sulfonic acid were added to the reaction liquid, and the reaction liquid mixture was stirred until the coupling reaction was completed. Finally, add sodium chloride (NaCl) for salting out, and take out by filtration to obtain the compound of the following formula (I-1).
[0061]
[0062] Then take 32.5 parts of 1-aminobenzene-2-methoxy-5-methyl-4-β-sulfate radical ethanesulfonyl (1-aminobenzene-2-methoxy-5-methyl-4-β-sulfatoethylsulfone) Dissolve and disperse in 1000 parts of ice water, add 24 parts of 32% hy...
preparation example 2
[0065] Take 36.1 parts of 1-aminobenzene-2-sulfonicacid-4-β-sulfato-ethylsulfone, dissolve and disperse in 1000 parts of ice water, add 24 parts of 32% hydrochloric acid aqueous solution and stir fully, then add 7.0 parts of sodium nitrite aqueous solution, and then Stirring was continued at 0-5°C until the diazotization was complete. Then, 23.9 parts of 2-amino-5-hydroxynaphthalene-7-sulfonic acid were added to the reaction liquid, and the reaction liquid mixture was stirred until the coupling reaction was completed. Finally, sodium chloride is added for salting out, and the mixture is taken out by filtration to obtain the compound of the following formula (I-2).
[0066]
[0067] Take 34.1 parts of 1-aminobenzene-2,5-dimethoxy-4-β-sulfate ethylsulfonyl (1-aminobenzene-2,5-dimethoxy-4-β-sulfatoethylsulfone) dissolved in 1000 parts In ice water, add 24 parts of 32% hydrochloric acid aqueous solution and stir thoroughly, then add 7.0 parts of sodium nitrite aqueous solution...
preparation example 3
[0070] Take 28.1 parts of 1-aminobenzene-4-β-sulfatoethylsulfonyl (1-aminobenzene-4-β-sulfatoethylsulfone) dissolved in 1000 parts of ice water, add 24 parts of 32% hydrochloric acid aqueous solution and stir thoroughly, Then add 7.0 parts of sodium nitrite aqueous solution, and then keep stirring at a temperature of 0-5° C. until the diazotization is completed. Then, 23.9 parts of 2-amino-5-hydroxynaphthalene-7-sulfonic acid were added to the reaction liquid, and the reaction liquid mixture was stirred until the coupling reaction was completed. Finally, sodium chloride is added for salting out, and the mixture is taken out by filtration to obtain the compound of the following formula (I-3).
[0071]
[0072] Take 34.1 parts of 1-aminobenzene-2, 5-dimethoxy-4-β-sulfato-ethylsulfone, dissolve and disperse in 1000 parts of ice water, add 24 parts of 32% hydrochloric acid aqueous solution and stir fully, then add 7.0 parts of sodium nitrite aqueous solution, and then Stirring...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com