Polymer containing calixarene, preparing method and use thereof
A technology for calixarene and polymer, which is applied in the fields of calixarene-containing polymers, preparation and use thereof, can solve the problems of little knowledge of the properties of calixarene derivatives, and achieve the effects of excellent solubility and enhanced electrical conductivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
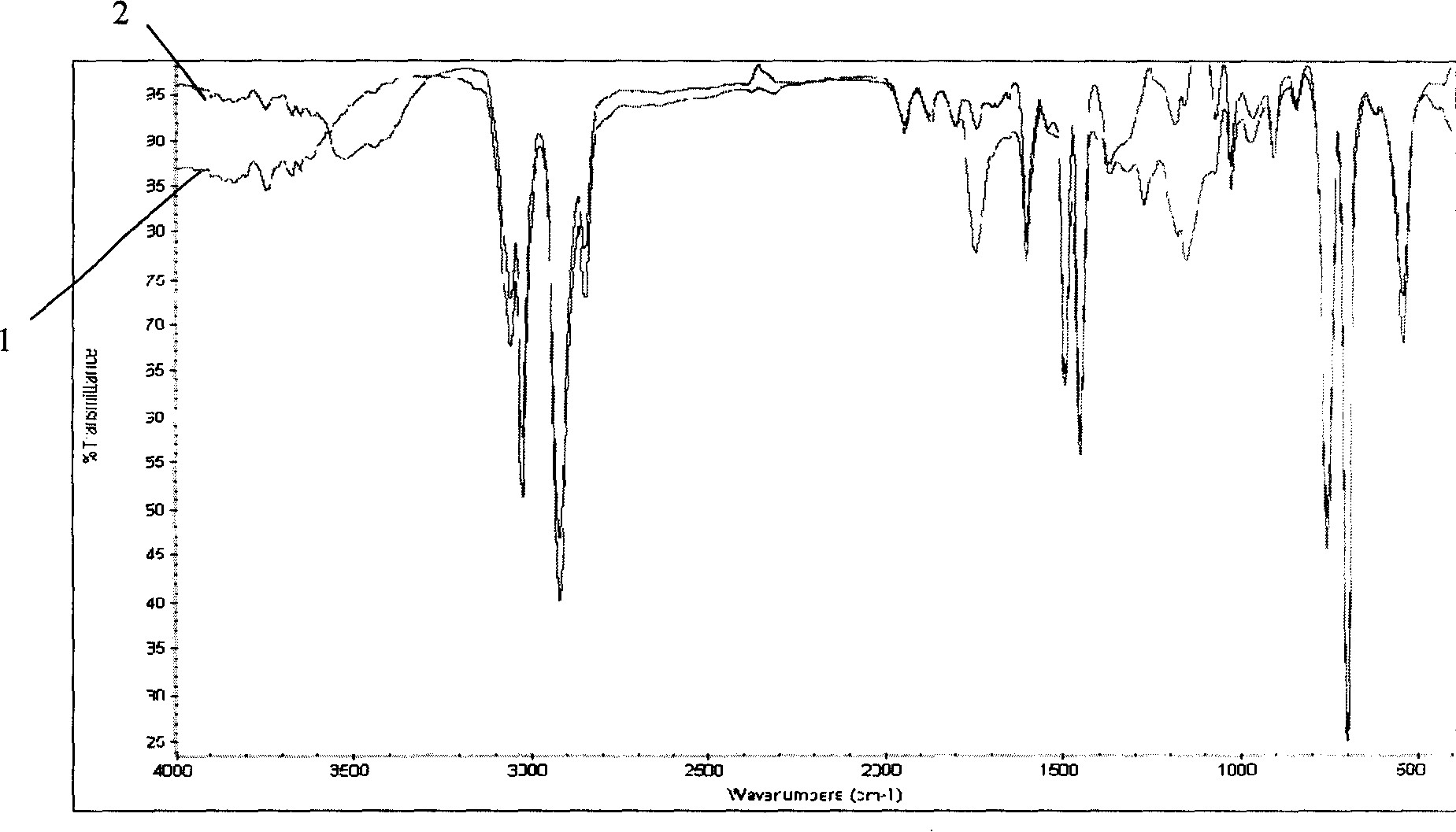
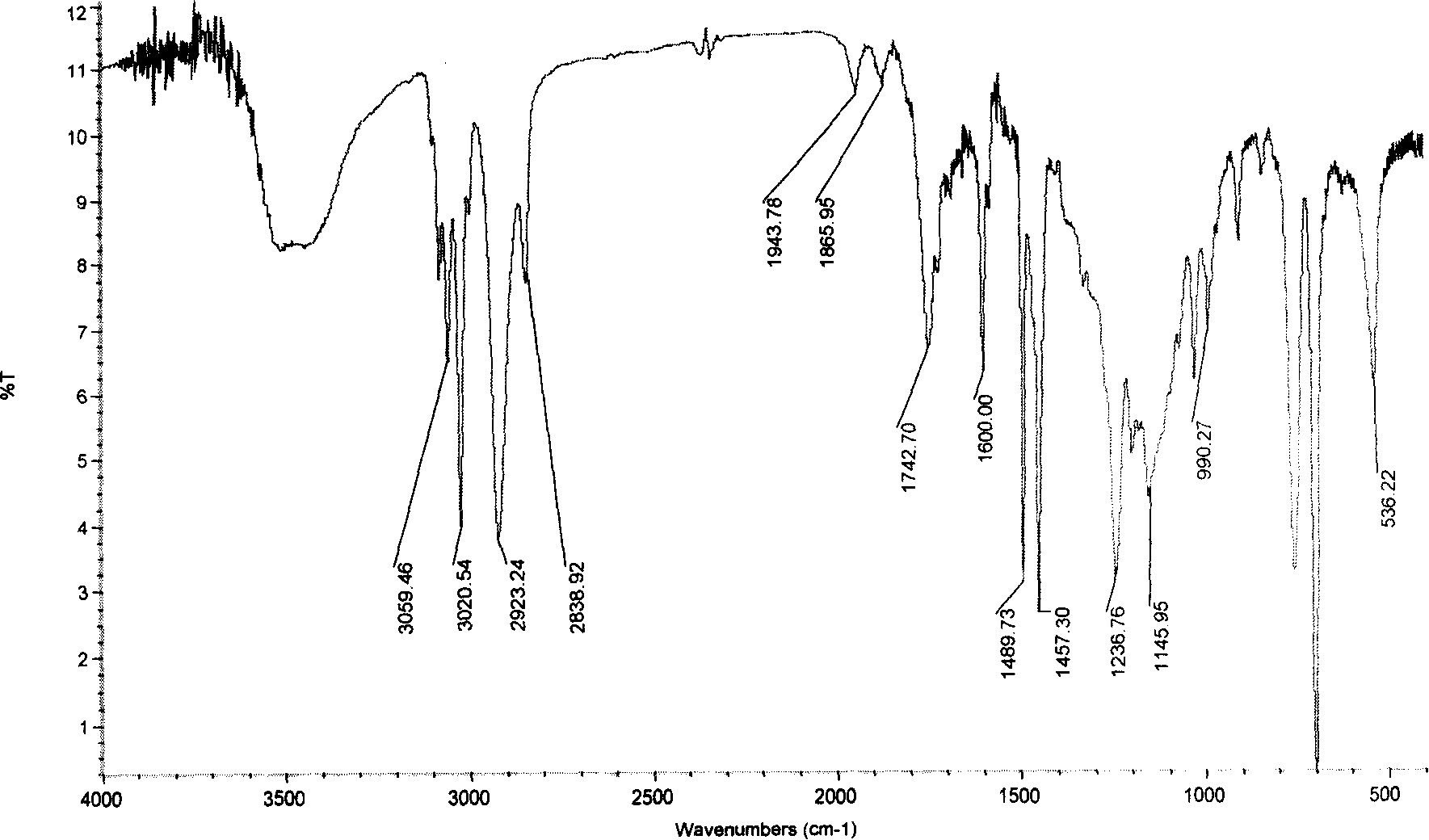
Image
Examples
Embodiment 1
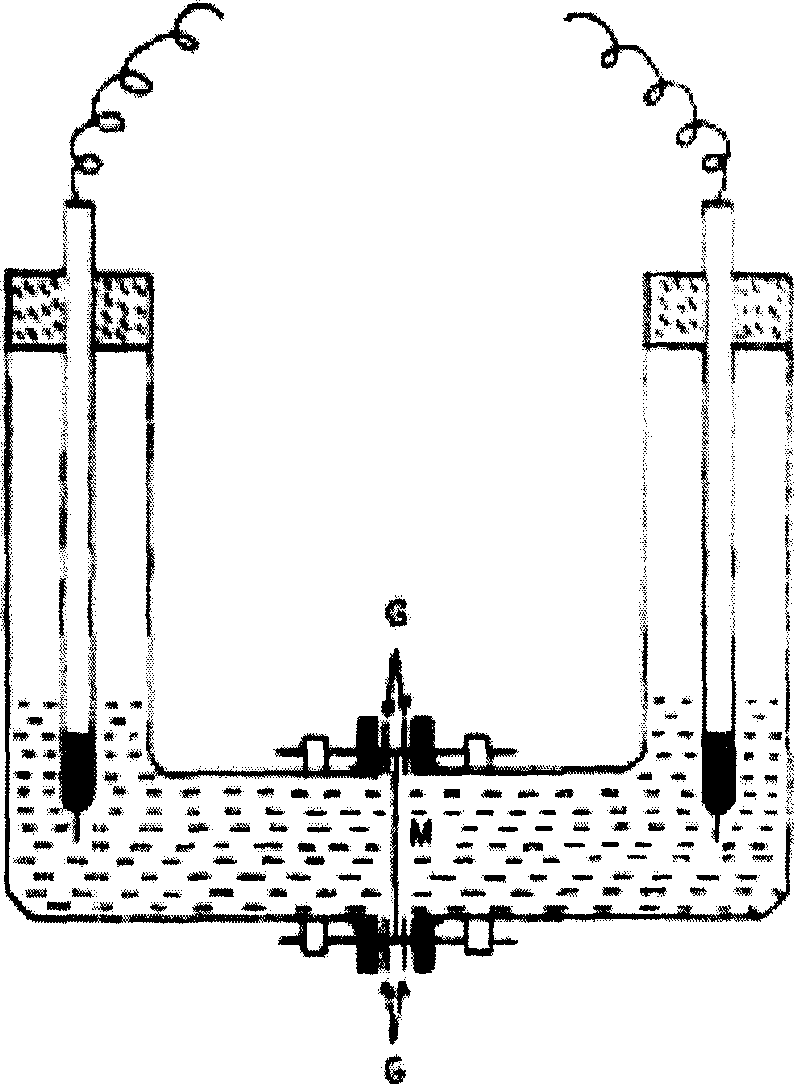
[0059] A. 2L stainless steel pressure-resistant reaction kettle, after pressure leak test, filled with argon gas and vacuumed three times, then set aside;
[0060] B. Dissolve 10g of 5-acrylamido-25,26,27,28-tetrahydroxycalix[4]arene in 50mL of chloroform for use;
[0061] C. Dissolve 30mL of styrene monomer in 50mL of chloroform, add 80mg of BPO under stirring, wait for all to dissolve, put it into the reaction kettle of step A, then pass argon gas into the reaction kettle to normal pressure, and stir , the rotating speed is 500 rpm; the reaction kettle is slowly heated to 70 ° C, and refluxed for 2 hours, and then the solution prepared in step B is injected with argon pressure to keep the pressure in the reaction kettle at 2 kg of atmospheric pressure; after continuing to reflux for 1 hour, press Put tetrafluoroethylene monomer, make the pressure in the reaction kettle reach 4-5 kilograms of atmospheric pressure; After stirring for 48 hours, wait for the pressure in the kett...
Embodiment 2
[0068] A. 2L stainless steel pressure-resistant reaction kettle, after pressure leak test, filled with argon gas and vacuumed three times, then set aside;
[0069] B. Dissolve 10g of 5-acrylamido-25,26,27,28-tetrahydroxycalix[4]arene in 50mL of chloroform for use;
[0070] C. Dissolve 70mL of styrene monomer in 150mL of chloroform, add 80mg of BPO under stirring, wait for all to dissolve, put it into the reaction kettle of step A, then pass argon gas into the reaction kettle to normal pressure, and stir , rotating speed 500 rev / min; Slowly heat up the reactor to 70°C, reflux for 2 hours, then inject the solution prepared in step B with argon pressure to keep the pressure in the reactor at 1.5 kg atmospheric pressure; continue to reflux for 1 hour, press Add vinylidene fluoride monomer to make the pressure in the reaction kettle reach 5 kilograms of atmospheric pressure; after stirring for 48 hours, wait for the pressure in the kettle to drop to a constant value, stop stirring,...
Embodiment 3
[0075] A. 2L stainless steel pressure-resistant reaction kettle, after pressure leak test, filled with argon gas and vacuumed three times, then set aside;
[0076] B. Dissolve 10g of 5-acrylamido-25,26,27,28-tetrahydroxycalix[4]arene in 50mL of chloroform for use;
[0077] C. Dissolve 90ml of styrene monomer in 150ml of chloroform, add 80mg of BPO under stirring, wait for all to dissolve, put it into the reaction kettle of step A, then pass argon gas into the reaction kettle to normal pressure, and stir , the rotating speed is 500 rpm; the reaction kettle is slowly heated to 70 ° C, and refluxed for 2 hours, and then the solution prepared in step B is injected with argon pressure to keep the pressure in the reaction kettle at 2 kg of atmospheric pressure; after continuing to reflux for 1 hour, press Put into chlorotrifluoroethylene monomer, make the pressure in the reactor reach 5 kilograms of atmospheric pressure; After stirring for 48 hours, treat that the pressure in the st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com