Peptide-based influenza vaccine formulation
A preparation and vaccine technology, applied in the field of peptide-based influenza vaccine preparations, to achieve broad protection and improve humoral response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0166] In this example, B6 mice were immunized with INF-01P vaccine plus Alum, Ribi or Montanide, or commercial vaccine (2004-2005 season). Sera from vaccinated mice were obtained one day before virus challenge. Mice were challenged with pathogenic A / HK / 1 / 68-MA20c virus, followed by three weeks of follow-up challenges.
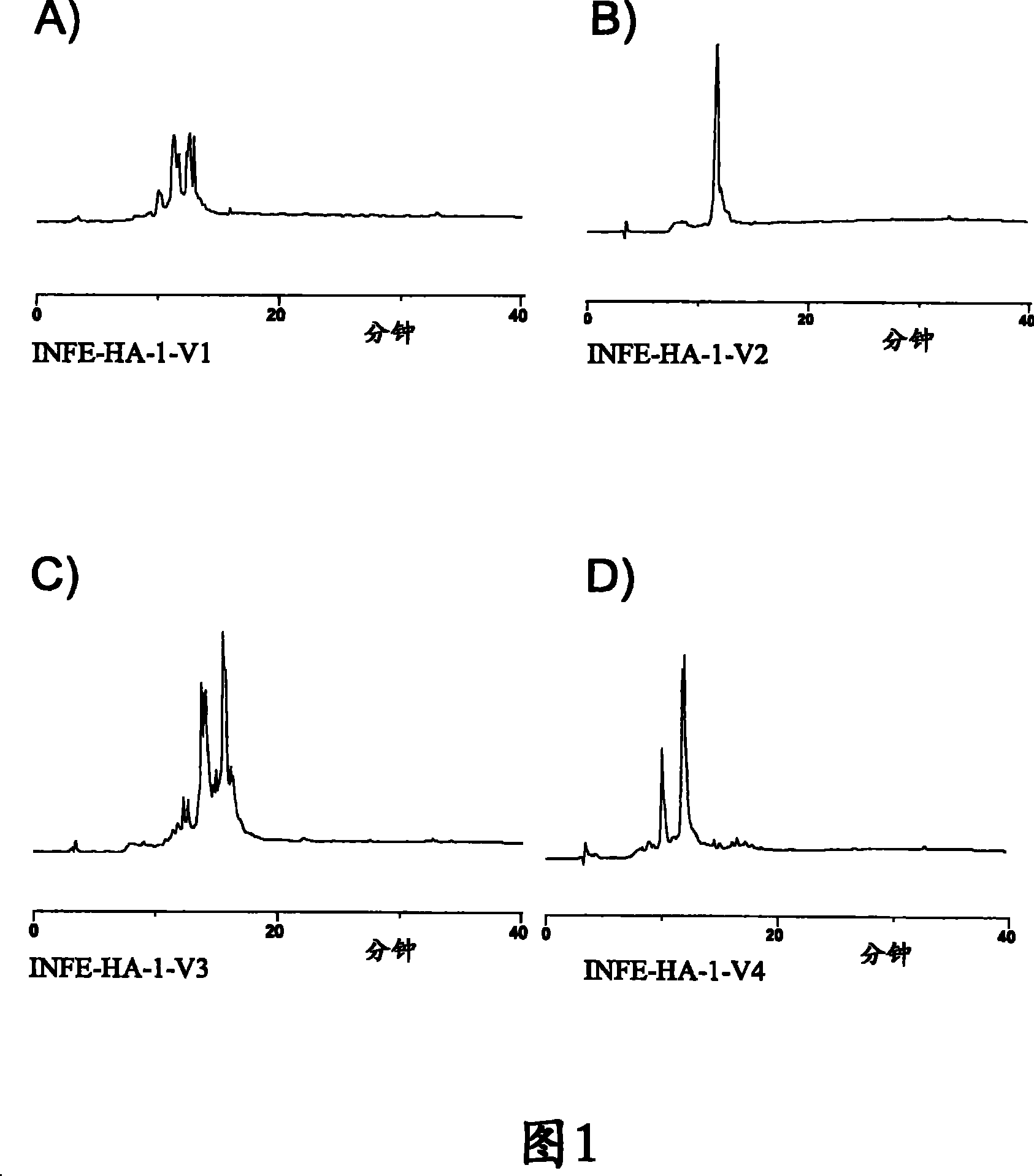
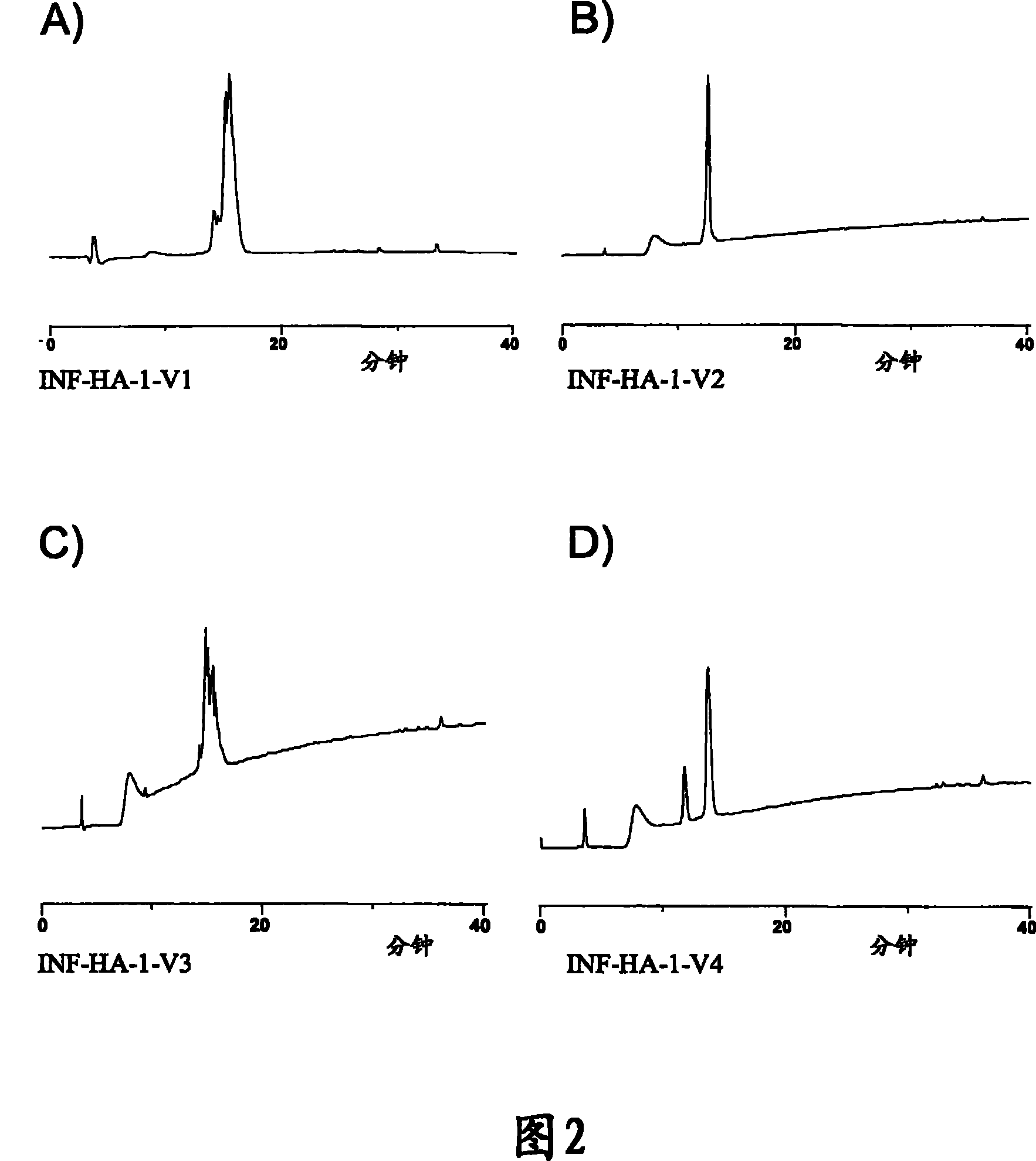
[0167] The INF-01P vaccine is based on the 4 human influenza sequence discontinuity construct formulations as shown in Table 19.
[0168] Table 19: INF-01P Influenza Vaccine Formulations
[0169] INF-HA-1-V1 (SEQ ID NO: 1)
YACKRGGKSSGSSYPVLNVSY (SEQ ID NOS: 1-16)
---H------------S-TM
INF-HA-1-V2 (SEQ ID NO: 17)
KKGSVHHPSTITEQTSLYVNA (SEQ ID NOS: 17-32)
-S-------------T--QQ-
[0170] INF-HA-1-V3 (SEQ ID NO: 33)
DVLFSVESPNNKNKDPIDTCD (SEQ ID NOS: 33-48)
------K-V-----ES-----
INF-HA-1-V4 (SEQ ID NO: 49)
YVSVSTSRIASRPKVRGQSGR (SEQ ID NOS: 49-64)
--T--S---G---W--------
[0171] Figure 9 Hu...
Embodiment 2
[0175] B6 mice were immunized with INFE-01P (equine influenza) vaccine plus alum or commercial vaccine (2004-2005 season). Test mouse sera against several influenza strains (H3N2 A / Hong Kong / 1 / 68g, H1 N1 A / FM / 1 / 47, H5N1 A / Hong Kong / 213 / 2003, B / Mass / 3 / 66 and H1 N1 A / NewCaledonia / 20 / 1999) HAI activity. Sera were obtained after the first vaccination.
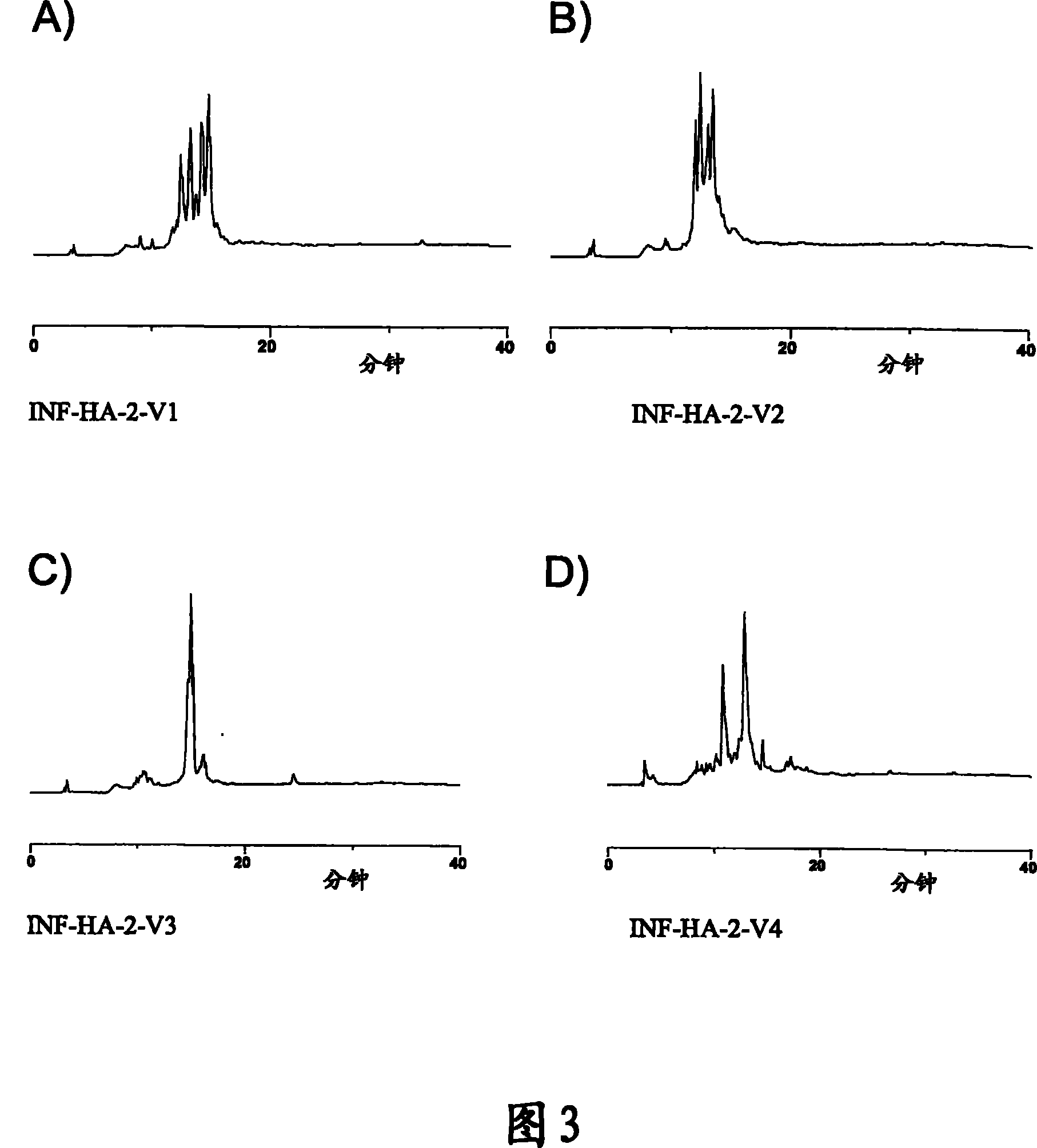
[0176] INFE-01P vaccine is based on 4 equine influenza sequence discontinuous site construct preparations as shown in Table 20:
[0177] Table 20: INFE-01P Equine Influenza Vaccine Formulations
[0178] INFE-HA-1-V1 (SEQ ID NO: 185)
[0179] Figure 12 Humoral immunity in mice immunized with INFE-01P, as measured by HAI titers, is demonstrated. As shown, humoral immunity against several strains of influenza virus was induced in mice immunized with this exemplary equine vaccine formulation compared to mice vaccinated with commercial vaccine or adjuvant alone (control).
Embodiment 3
[0181] Hemagglutination (HAI) assay
[0182] The immunogenicity of individual or combined discontinuous epitope constructs was evaluated in mice. Mice immunized together with the four discontinuous epitope constructs developed antibodies capable of inhibiting the hemagglutination reactivity of the virus. Influenza-based discontinuous epitope constructs were shown to successfully mimic the discontinuous epitope as antibodies were elicited that inhibit hemagglutination caused by influenza virus.
[0183] Induction of functionally relevant antibodies against HA was measured using a standard HAI assay. The HAI titer induced by a candidate vaccine formulation was tested using a variety of different influenza strains to determine the extent of immunity induced by the vaccine formulation.
[0184] Figure 13 Shown are the results of hemagglutination assays performed in the murine vaccine study. Each vaccine group received a different vaccine formulation or phosphate buffered sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com