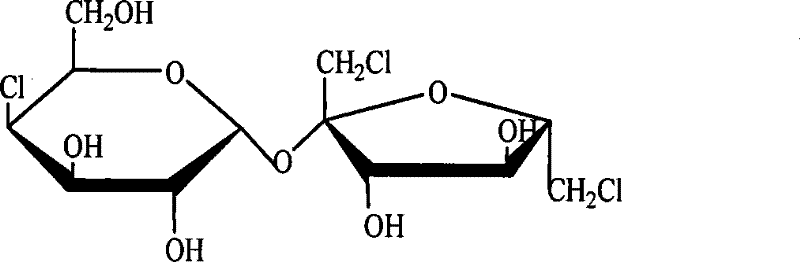
Sucralose intermediate analysis detection method
A technology of sucralose and detection method, which is applied in the food field and can solve the problems of low sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
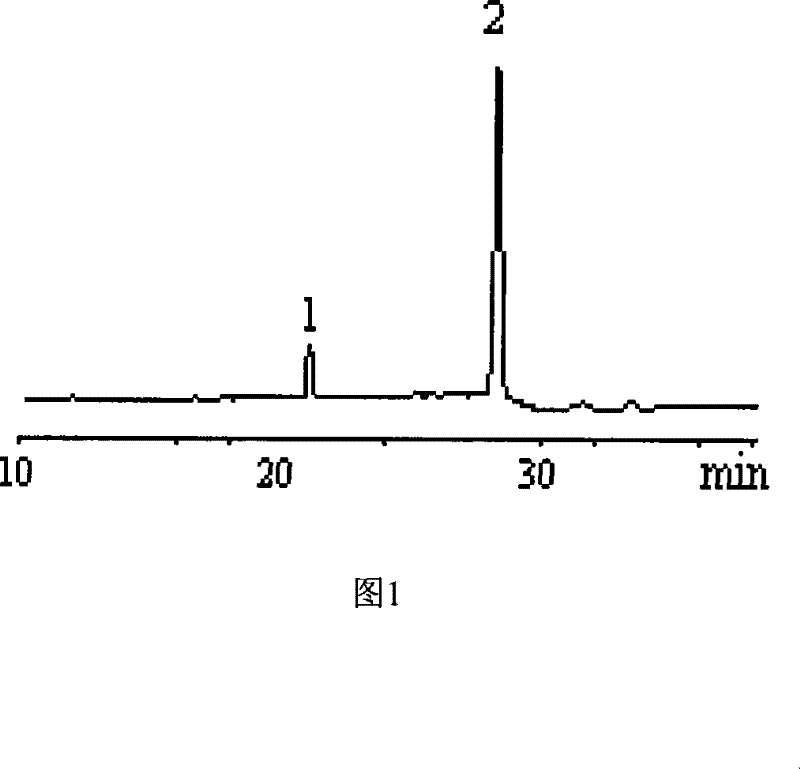
[0017] Dissolve 10 mg of standard 4-PAS or 6-PAS in 1 ml of pyridine, add 0.4 ml of hexamethyldisilazane and 0.2 ml of trimethylchlorosilane, react for five minutes, evaporate to dryness under reduced pressure, add dichloromethane to dissolve, Centrifuge and take the supernatant for GC and GC analysis. The retention time of 4-PAS was 21.164min, and the retention time of 6-PAS was 28.455min.
[0018] Chromatographic conditions: analyzed by Shimadzu GC-2010 gas chromatograph. Chromatographic column: rtx-50, 30m×0.25mm×0.25μm; inlet temperature: 270°C; detector temperature: 280°C; column oven temperature: 250°C; carrier gas is N 2 , flow rate is 0.6ml / min; split ratio: 19:1.
[0019] Gas conditions: Shimadzu GCMS-QP2010 gas chromatography-mass spectrometer was used. Chromatographic column: rtx-5ms, 30m×0.25mm×0.25μm; the temperature of the injection port, detector and column thermostat is consistent with the above gas phase conditions. The carrier gas is He, the flow rate is ...
Embodiment 2
[0021] With reference to embodiment 1 chromatographic conditions.
[0022] Take 10 mg of the synthesized sucralose intermediate sample containing 4-PAS and dissolve it in 1 ml of pyridine, add 0.4 ml of hexamethyldisilazane and 0.2 ml of trimethylchlorosilane, react for five minutes, evaporate to dryness under reduced pressure, add Dichloromethane was dissolved, centrifuged, and the supernatant was taken for gas chromatography and gas chromatography analysis (the retention time of 4-PAS was 21.164min), and the content of 4-PAS obtained quantitatively by the internal standard method was 82%.
Embodiment 3
[0024] With reference to embodiment 1 chromatographic conditions.
[0025] Samples were taken during the reaction of 4-PAS to generate 6-PAS, evaporated to dryness under reduced pressure, dissolved in 1ml of pyridine, added 0.4ml of hexamethyldisilazane and 0.2ml of trimethylchlorosilane, reacted for five minutes, evaporated under reduced pressure Dry, add dichloromethane to dissolve, centrifuge, get the supernatant and carry out gas chromatography analysis (the retention time of 4-PAS is 21.164min; The retention time of 6-PAS is 28.455min), adopt the area normalization method to just can determine 4 The relative content of -PAS and 6-PAS, so that the reaction conditions can be optimized.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com