Methods of dosing propofol prodrugs for inducing mild to moderate levels of sedation
A mild, sedative technology, used in pharmaceutical formulations, drug combinations, medical preparations containing active ingredients, etc., can solve problems such as hindering development and poor bioavailability of propofol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
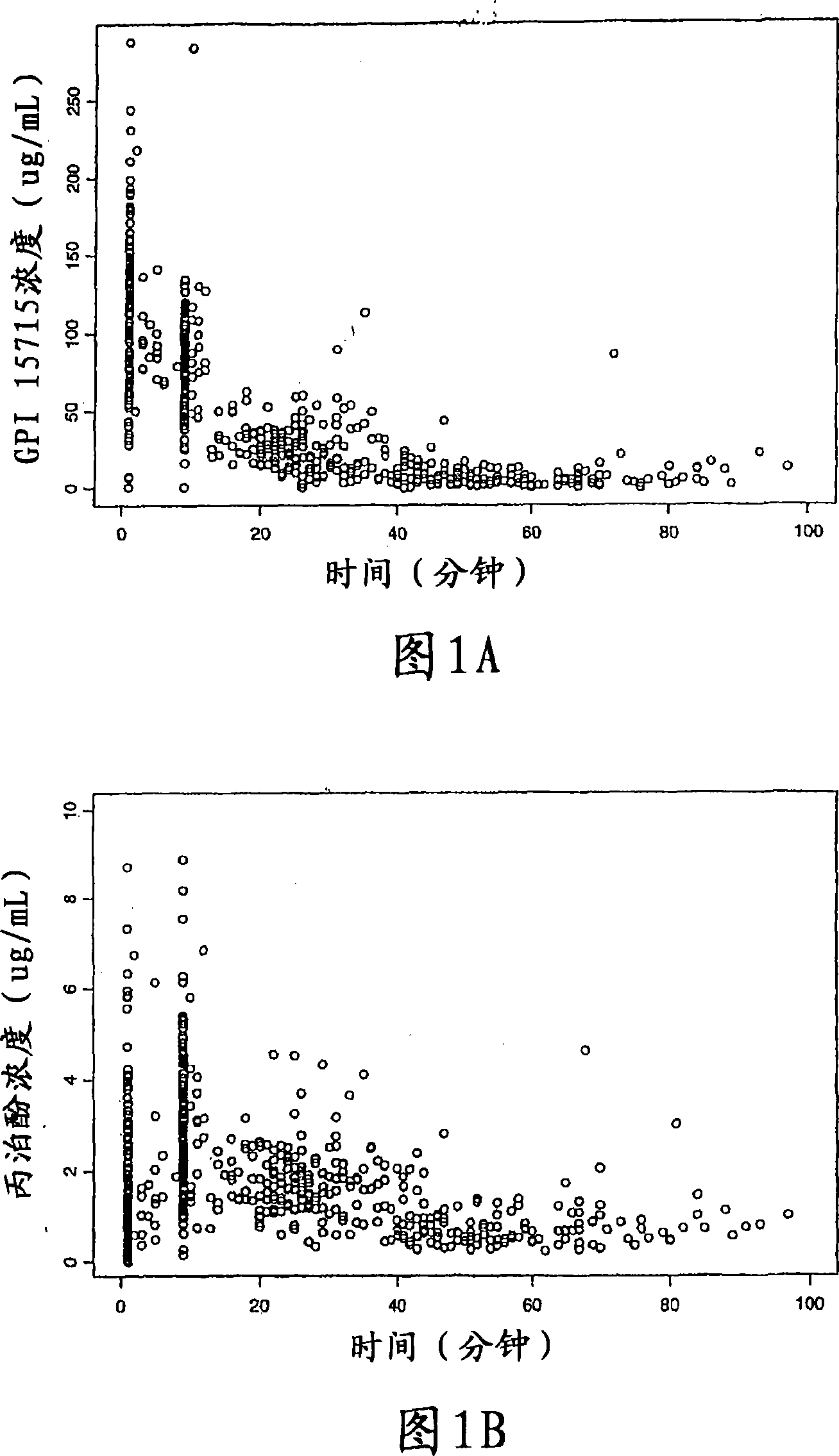
[0053] In this example, 93 patients were administered the fentanyl and bolus propofol prodrug doses as summarized in Table 2 below.
[0054] Table 2
[0055] Propofol prodrug rapid injection dose group
[0056] 7.5 mg /
10mg / thousand
12.5 mg /
Total
Quantitative
0.5 μg / kg
4
13
10
27
1.0 μg / kg
9
14
11
34
1.5 μg / kg
11*
10
11
32
Total
24
37
32
93
[0057] *One patient received 1.5 μg / kg fentanyl + 8.5 μg / kg bolus dose of propofol prodrug
[0058] Demographics of patients in Example 1A summarized by original assignment to treatment groups (mg / kg) in Table 3.
[0059] Table 3 Demographic and other baseline characteristics of treatment groups (Example 1A)
[0060] Rapid Injection Do...
Embodiment 1B
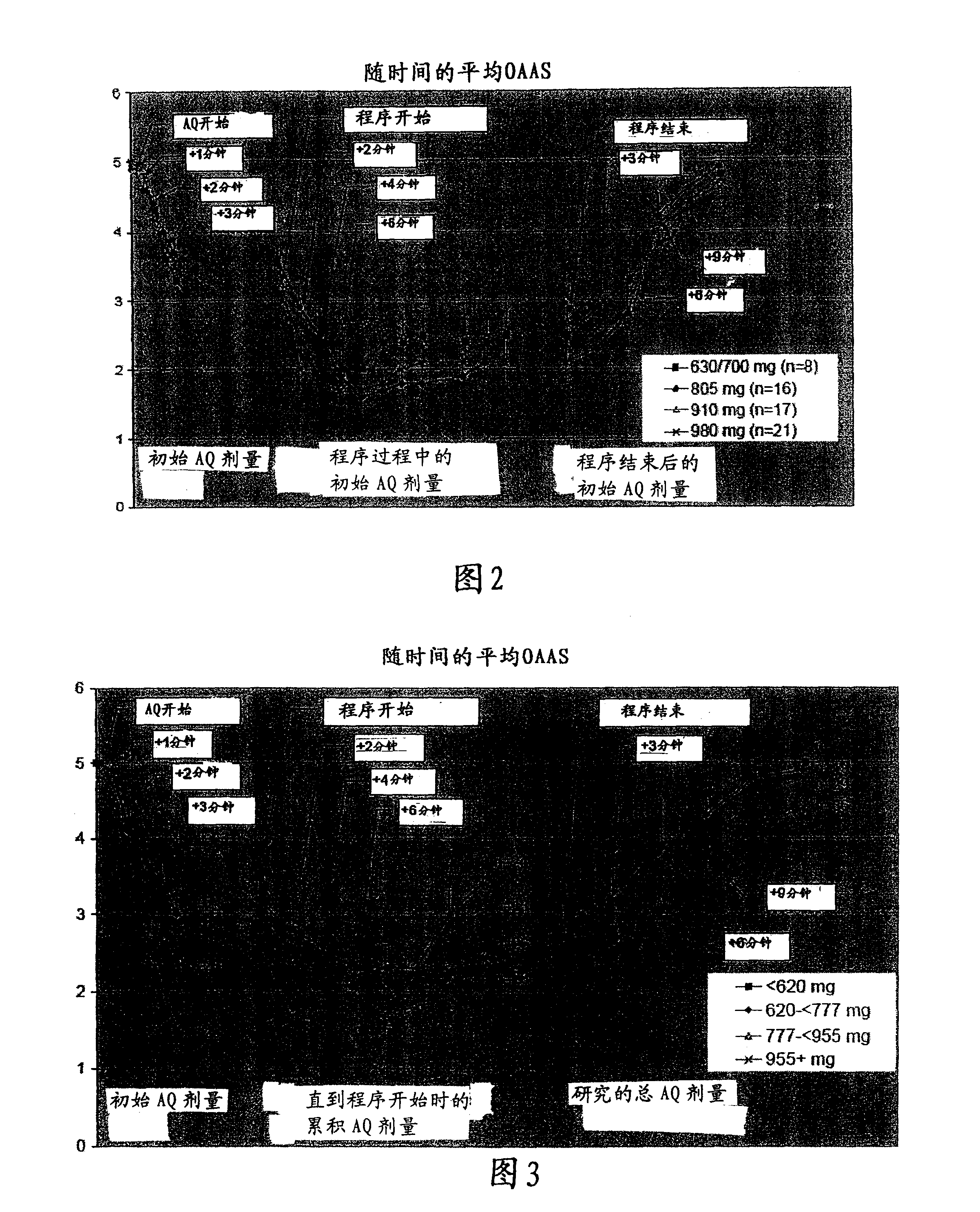
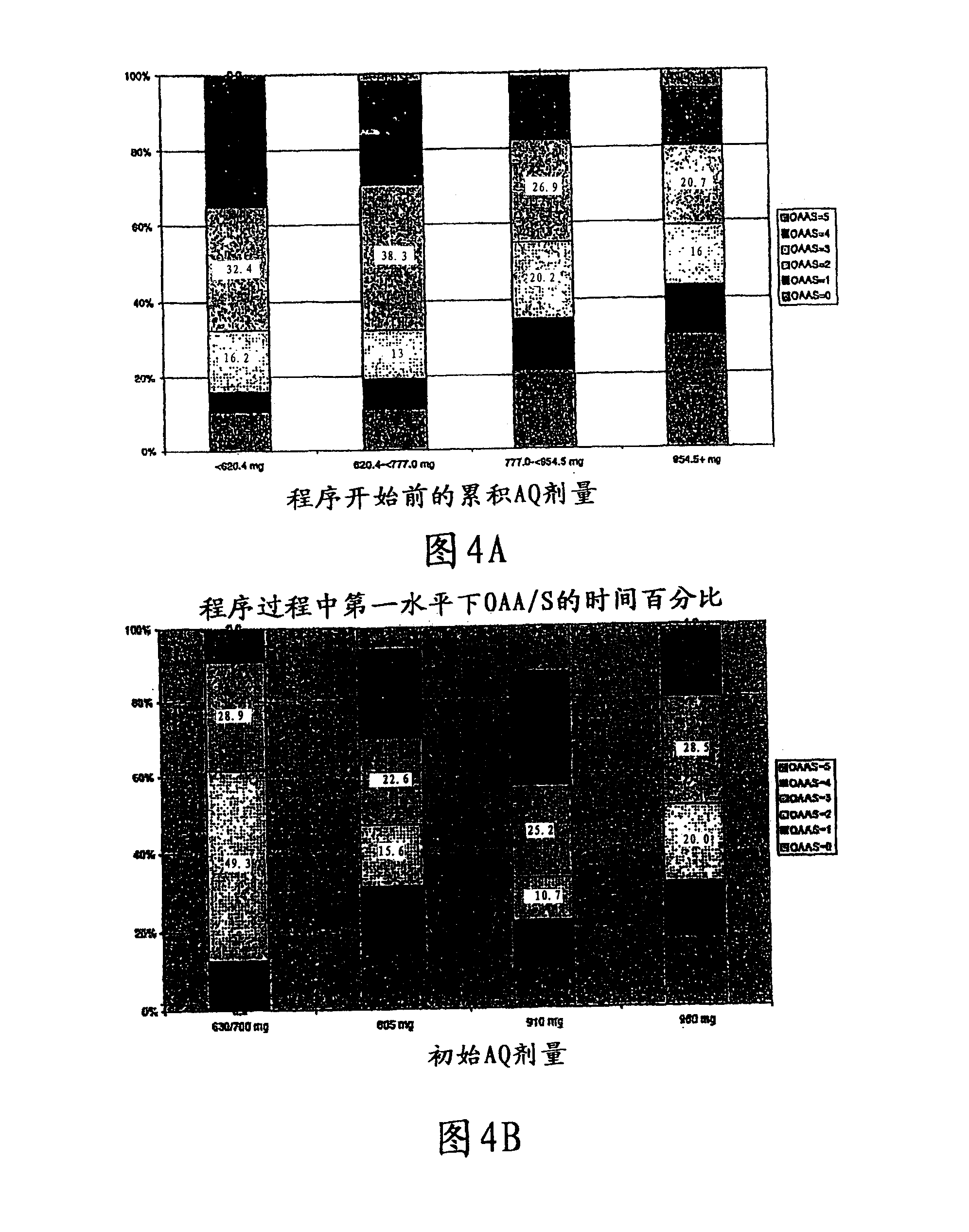
[0070] In this example, the initial dosing regimen was divided into 2 individual reconstitutions, each with fixed fentanyl and propofol prodrug doses. Some initial patients weighed 75 to 80 kg and were dosed with 980 mg (28 mL). During this initial portion, subjects became increasingly sedated (MOAA / S75 to >80 kg. Table 5 summarizes the adjusted weight-based fixed dosing regimen.
[0071] Table 5 Weight-Based Fixed Dosing Regimen
[0072] patient weight
Pretreatment Fen
Dosage of tanyl
Initial propofol prodrug rapid
injection dose
Propofol Prodrug Supplement Dosage
18-70 years old
≤50kg
50 micrograms
630 mg (18 mL)
Up to 105 mg (3 mL)
>50kg to ≤65kg
60 micrograms
700mg (20ml)
Up to 140 mg (4 mL)
>65kg to ≤80kg
70 micrograms
805 mg (23 mL)
Up to 175 mg (5 mL)
>80kg
80 micrograms
910 mg (26 mL)
Up to 175 mg (5 mL)
Patients ≥70 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com