Dosages for treatment with anti-egfr antibodies
A kind of antibody, technology of use, application in the preparation field for anti-ErbB2 antibody treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0289] Controlled release formulations may also be prepared. Suitable examples of controlled release formulations include semipermeable matrices of solid hydrophobic polymers containing the antibody in shaped articles such as membranes or microcapsules. Examples of controlled release formulations include polyesters, hydrogels such as poly(2-hydroxyethyl-methacrylate) or poly(vinyl alcohol), polylactide (US Patent 3,773,919), L-glutamic acid and gamma ethyl - Copolymers of L-glutamate, non-degradable ethylene ethyl acetate, degradable lactic acid-glycolic acid copolymers such as LUPRON DEPOT TM (injectable microspheres composed of lactic-co-glycolic acid and leuprolide acetate), and poly-D-(-)-3-hydroxybutyric acid. While polymers such as ethylene-vinyl acetate and lactic acid-glycolic acid can release molecules for more than 100 days, certain hydrogels release proteins for a shorter period of time. When encapsulated antibodies stay in the body for a long time, they can denat...
Embodiment 1
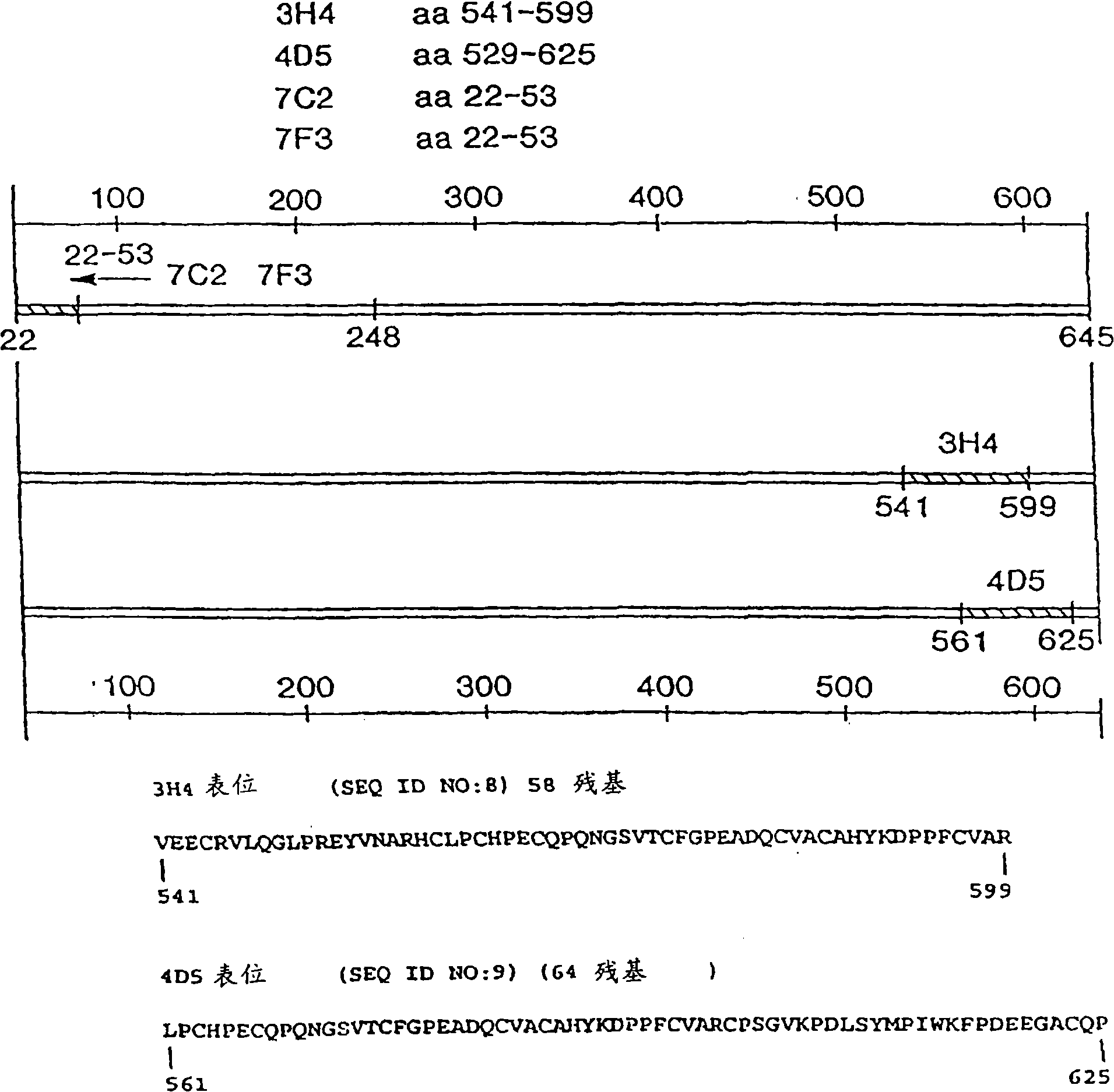
[0318] Example 1: HERCEPTIN Preparation and potency of anti-ErbB2 antibodies
[0319] Materials and Methods
[0320] Anti-ErbB2 monoclonal antibody According to the methods described in Fendly et al., Cancer Research 50: 1550-1558 (1990) and WO89 / 06692, anti-ErbB2 IgG specifically binding to the extracellular domain of ErbB2 was prepared 1 Kappa mouse monoclonal antibody 4D5. Briefly, NIH 3T3 / HER2-3 prepared as described by Hudziak et al. (Proc. 400 cells (approximately expressing 1×10 5 ErbB2 molecule / cell), and immunized BALB / c mice with it. At 0, 2, 5 and 7 weeks, mice were intraperitoneally injected with 10 7 0.5ml PBS for each cell. At weeks 9 and 13, specific antiserum (which can be compared with 32 P-labeled ErbB2 immunoprecipitation) mice were intraperitoneally injected with wheat germ agglutinin-Sepharose (WGA) purified ErbB2 membrane extracts. Afterwards 0.1 ml of ErbB2 preparation was injected intravenously, and the splenocytes were fused with the mou...
Embodiment 2
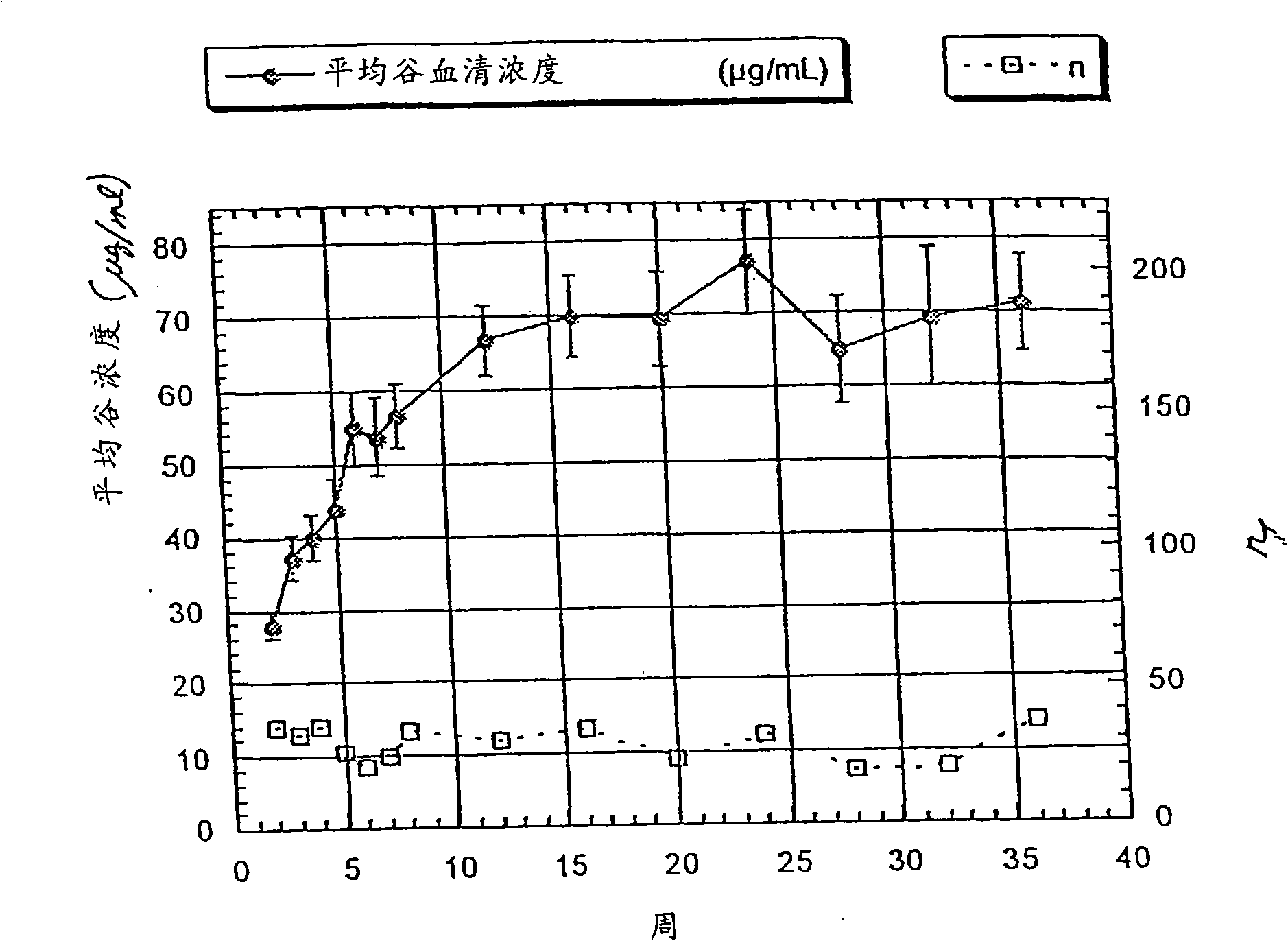
[0370] Embodiment 2: Anti-ErbB2 antibody (HERCEPTIN ) pharmacokinetic and pharmacodynamic properties
[0371] HERCEPTIN was administered via intravenous infusion to patients selected according to the criteria provided in Example 1 Anti-ErbB2 antibody. HERCEPTIN at an initial dose of 4 mg / kg by intravenous infusion Anti-ErbB2 antibody, followed by HERCEPTIN 2 mg / kg IV once a week for several weeks Anti-ErbB2 antibody. Of the 213 patients who started the regimen, fewer than 90 had blood levels collected over 8 weeks due to selective discontinuation of patients with rapidly progressive disease. Among the 213 patients who started treatment, the number of people whose serum trough concentration could be measured at different times was: 80 at 12 weeks, 77 at 16 weeks, 44 at 20 weeks, 51 at 24 weeks, and 51 at 28 weeks 25 at 32 weeks, 23 at 32 weeks and 37 at 36 weeks.
[0372] HERCEPTIN Trough serum concentrations of anti-ErbB2 antibodies at weeks 0-36
[0373] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com