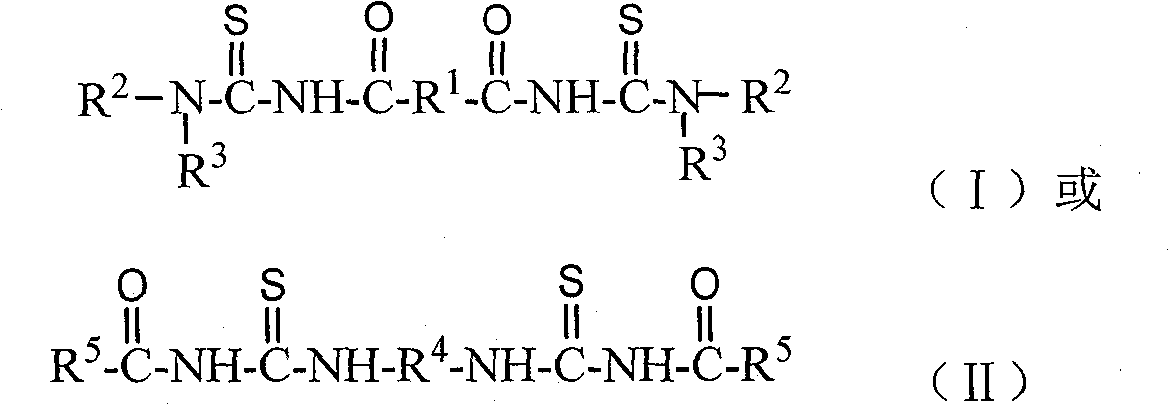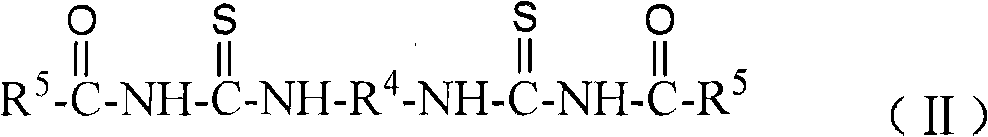Sulphide ore floation collector and use method of diacyl bis-thiourea and preparation method thereof
A diacylbisthiourea, application method technology, applied in flotation, organic chemistry, solid separation and other directions, to achieve the effects of good selectivity, simple preparation process, and high-efficiency flotation separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
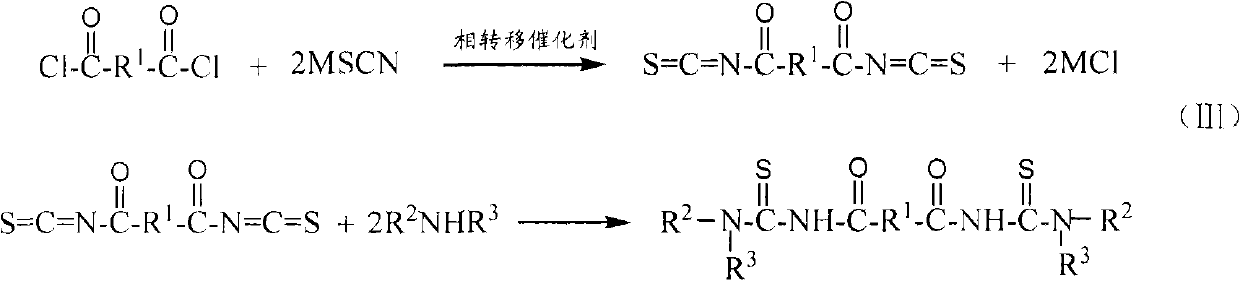
[0038] Embodiment 1: N, N'-dipropyl-N ", the synthesis of N " '-(terephthaloyl) bis (thiourea)
[0039] In a 250ml three-necked flask, add 2.03g of terephthaloyl chloride (0.01mol), 3 drops of PEG-400 (about 0.2g), 2.46g of potassium thiocyanate (0.025mol) and 40ml of dichloromethane, and stir at 20°C After reacting for 3.5 hours, a yellow reaction liquid containing a terephthaloyl diisothiocyanate intermediate was obtained. Then, at 20°C, slowly add 10 ml of dichloromethane solution dissolved with 1.68 mL of n-propylamine (0.02 mol) dropwise to the above-mentioned three-necked flask containing the intermediate of terephthaloyl diisothiocyanate, and continue React at 20°C for 1 hour to obtain a reaction solution containing N,N'-dipropyl-N",N"'-(terephthaloyl)bis(thiourea). The reaction solution was suction-filtered to remove by-product potassium chloride, dichloromethane was evaporated under reduced pressure to obtain N, N'-dipropyl-N", N"'-(terephthaloyl)bis(thiourea ) init...
Embodiment 2
[0040] Embodiment 2: N, N'-dibutyl-N ", the synthesis of N " '-(terephthaloyl) bis (thiourea)
[0041] In a 250ml three-necked flask, add 2.03g of terephthaloyl chloride (0.01mol), 3 drops of PEG-400 (about 0.2g), 2.46g of potassium thiocyanate (0.025mol) and 40ml of dichloromethane, and stir at 15°C After reacting for 3.5 hours, a yellow reaction liquid containing a terephthaloyl diisothiocyanate intermediate was obtained. Then, at 15°C, slowly add 10 ml of dichloromethane solution dissolved with 2.01 mL of n-propylamine (0.02 mol) dropwise to the above-mentioned three-necked flask containing the intermediate of terephthaloyl diisothiocyanate, and continue React at 15°C for 1 hour to obtain a reaction solution containing N,N'-dibutyl-N",N"'-(terephthaloyl)bis(thiourea). The reaction solution was suction-filtered to remove by-product potassium chloride, dichloromethane was evaporated under reduced pressure to obtain N,N'-dibutyl-N",N"'-(terephthaloyl)bis(thiourea ) initial p...
Embodiment 3
[0042] Embodiment 3: N, N'-dipropyl-N ", the synthesis of N " '-(1,4-succinyl) bis(thiourea)
[0043] In a 250ml three-necked flask, add 1.55g 1,4-succinoyl chloride (0.01mol), 3 drops of PEG-400 (about 0.2g), 2.46g potassium thiocyanate (0.025mol) and 40ml dichloromethane, at 20°C The mixture was stirred and reacted for 3.5 hours to obtain a reaction solution containing 1,4-succinyl diisothiocyanate intermediate. Then at 20°C, slowly add 10ml of dichloromethane solution in which 1.68mL of n-propylamine (0.02mol) was dissolved into the above-mentioned three-necked flask containing 1,4-succinyl diisothiocyanate intermediate, dropwise The reaction was continued at 20°C for 1 hour to obtain a reaction liquid containing N,N'-dipropyl-N",N"'-(1,4-succinoyl)bis(thiourea). The reaction solution was suction filtered to remove the by-product potassium chloride, dichloromethane was evaporated under reduced pressure to obtain N, N'-dipropyl-N", N"'-(1,4-succinyl)bis( Thiourea) initial ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com