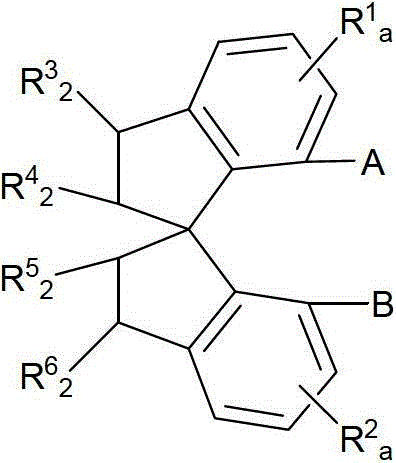
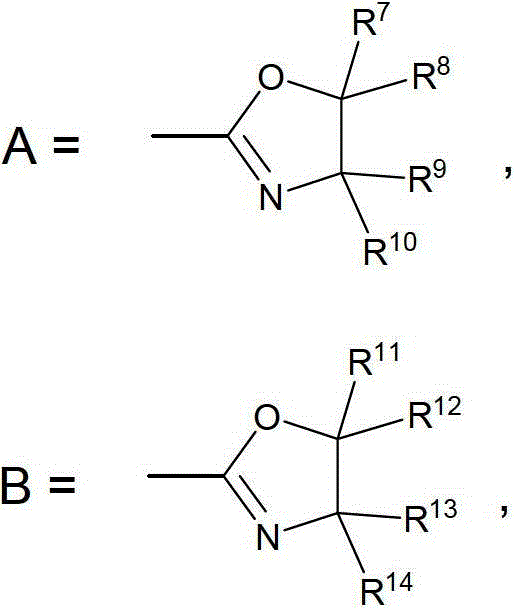
Novel chiral iron complex, and preparation and use thereof
An axial chirality and reactant technology, applied in the field of new chiral iron complexes, can solve the problem of lack of chiral iron complex catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Comparison of Iron Precursors
[0056]
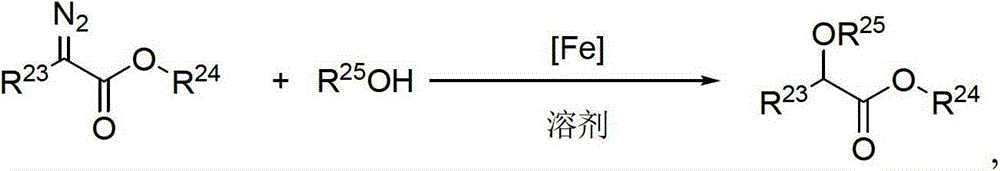
[0057] Weigh the iron precursor (0.015mmol), (S a , S, S)-1 (9.2mg, 0.018mmol), NaBArF (16.9mg, 0.018mmol), add 3ml of chloroform, and stir for complexation at room temperature for 4 hours. Add water (27mg, 1.5mmol) and stir for a while, then add a solution of methyl α-diazophenylacetate (52mg, 0.3mmol) and 1ml of chloroform, stir the reaction at 40°C, and monitor and track it by TLC until the reaction is complete. Column chromatography (PE / EA=5:1) gave the product as a colorless liquid. 1 HNMR (300MHz, CDCl 3 ): 7.43-7.33 (m, 5H), 5.18 (d, J=4.8Hz, 1H), 3.76 (s, 3H), 3.46 (d, J=4.8Hz, 1H).
[0058] The ee value of the product is determined by SFC, analysis conditions: Chiralcel OD-H column, sc CO 2 / i-PrOH=80:20, flow rate=2.0mL / min, detector wavelength=220nm, pressure=100bar, t R = 2.35min corresponds to (S)-isomer, t R = 2.87 min corresponds to the (R)-isomer.
[0059] The catalytic reactions of the cata...
Embodiment 2
[0062] Example 2: Ligand Comparison
[0063]
[0064] Weigh FeCl in the glove box 2 .4H 2 O (3.0mg, 0.015mmol), ligand (0.018mmol), NaBArF (16.9mg, 0.018mmol), 3ml of chloroform was added, and complexation was stirred at room temperature for 4 hours. Add water (27mg, 1.5mmol) and stir for a while, then add a solution of methyl α-diazophenylacetate (52mg, 0.3mmol) and 1ml of chloroform, stir the reaction at 40°C, and monitor and track it by TLC until the reaction is complete. Column chromatography (PE / EA=5:1) gave the product as a colorless liquid. Product NMR analysis data and ee value measuring method are with embodiment 1.
[0065] The catalytic reactions of the catalysts prepared by different ligands are shown in Table 2.
[0066] Table 2: Ligand Comparison
[0067]
[0068]
Embodiment 3
[0069] Embodiment 3: solvent comparison
[0070]
[0071] Weigh FeCl in the glove box 2 .4H 2 O (3.0mg, 0.015mmol), (S a , S, S)-1 (9.2mg, 0.018mmol), NaBArF (16.9mg, 0.018mmol), add 3ml of solvent, and stir complexation at room temperature for 4 hours. Add water (27mg, 1.5mmol) and stir for a while, then add a solution made of α-methyl diazophenylacetate (52mg, 0.3mmol) and 1ml of solvent, stir the reaction at 40°C, monitor and track by TLC until the reaction is complete, the silica gel column layer Analysis (PE / EA=5:1) gave the product as a colorless liquid. Product NMR analysis data and ee value measuring method are with embodiment 1.
[0072] The catalytic reactions of the catalysts prepared in different solvents are shown in Table 3.
[0073] Table 3: Solvent Comparison
[0074] experiment solvent Reaction time (h) Yield (%) Ee(%) 1 CHCl 3 10 90 94 2 CH 2 Cl 2 10 86 87 3 DCE 15 88 91
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com