Catalyst used for preparing methanol by hydrogenation of carbon dioxide and a preparation method thereof
A carbon dioxide and catalyst technology, which is applied in the field of palladium-zinc catalysts, can solve the problems that catalysts need to be improved urgently, and achieve the effects of active and stable, high activity, and easy production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 0.167g of PdCl 2 (purity is AR grade) dissolved with concentrated hydrochloric acid, put into a beaker filled with 50mL ethylene glycol (purity is AR grade), add 0.157g KOH (purity is AR grade) aqueous solution (concentration is 4mol / L), Maintain the pH value of the solution in the range of 4.5 to 5.0, stir for 30 minutes, then add 1.500 g of purified CNT, the feed solution is ultrasonically treated for 30 minutes, and then placed in a microwave oven (2450MHz, 640W); microwave radiation heating for 100s, cooling 20s, reheated for 10s, repeated cooling for 20s, reheated for 10s, took out the feed liquid and placed it in a cold water bath to let it cool rapidly, the feed liquid was filtered, and the filter was washed successively with acetone and deionized water until the filtrate was neutral. Dry at 110°C to obtain metal Pd-modified CNTs, whose stoichiometric formula is 5.0% Pd / CNT as determined by EDX analysis.
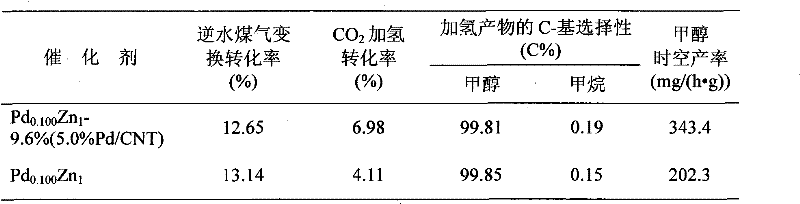
[0022] At 60°C, will contain 0.887g PdCl 2 (purity is A...
Embodiment 2
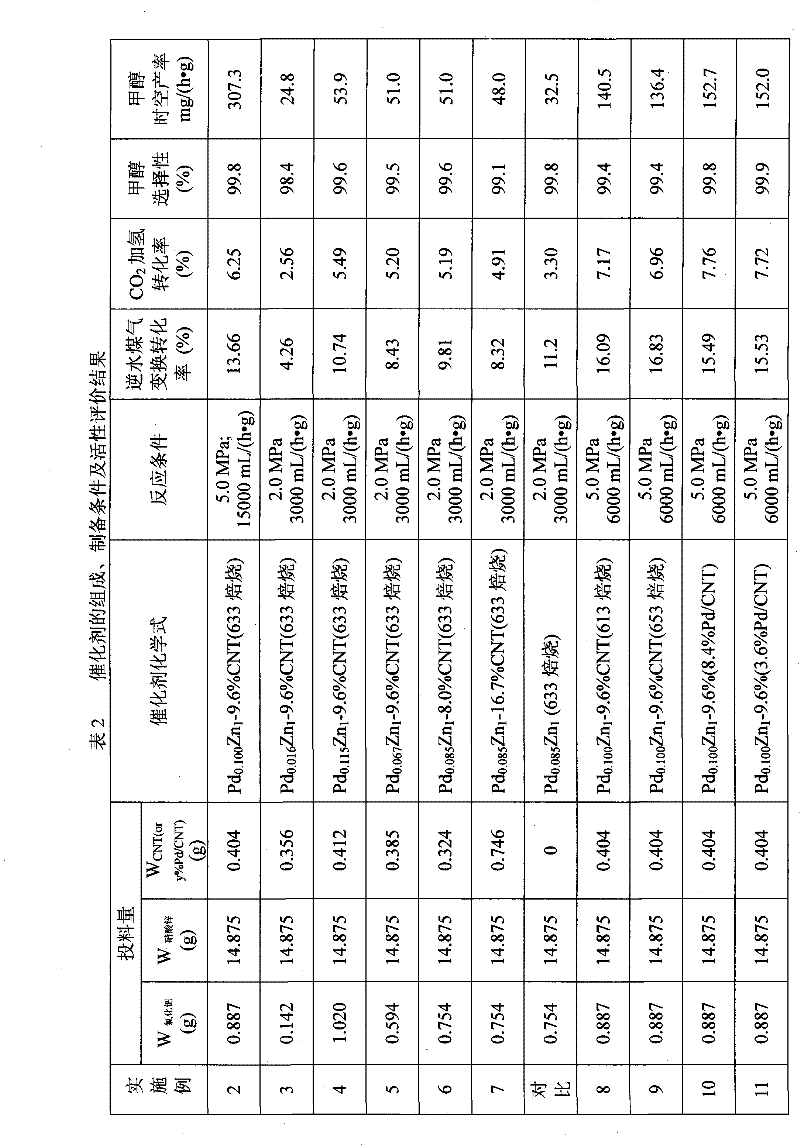
[0028] At 60°C, will contain 0.887g PdCl 2 (purity is AR grade) aqueous solution (concentration is 0.56mol / L) and contains 14.875g Zn(NO 3 ) 2 ·6H 2 After the aqueous solution (concentration of 1mol / L) of O (purity is AR grade) is mixed, it is quickly added dropwise to 140mL of Na with a concentration of 1mol / L. 2 CO 3 (Purity is AR grade) in a beaker of aqueous solution, carry out co-precipitation reaction under vigorous stirring, adjust Na 2 CO 3 The amount of solution added keeps the pH value of the reaction mixture at about 9.5, and the stirring is stopped after 30 minutes. The precipitate is centrifugally filtered, washed with deionized water until the filtrate is neutral, and then mixed with 0.404 g of CNT, beaten for 30 minutes, and centrifugally filtered again. The filter cake was dried at 110°C for 5 hours, pure N 2 Calcined at 360°C for 3 hours in the atmosphere, the stoichiometric formula is Pd 0.100 Zn 1 - 9.6% CNT catalyst (oxidation precursor state), pres...
Embodiment 3
[0031] At 65°C, will contain 0.142g PdCl 2 (purity is AR grade) aqueous solution (concentration is 0.56mol / L) and contains 14.875g Zn(NO 3 ) 2 ·6H 2 After the aqueous solution (concentration of 1mol / L) of O (purity is AR grade) is mixed, it is quickly added dropwise to 140mL of Na with a concentration of 1mol / L. 2 CO 3 (Purity is AR grade) in a beaker of aqueous solution, carry out co-precipitation reaction under vigorous stirring, adjust Na 2 CO 3 The amount of solution added keeps the pH value of the reaction mixture at about 9.5, and the stirring is stopped after 30 minutes. The precipitate is centrifugally filtered, washed with deionized water until the filtrate is neutral, and then mixed with 0.356 g of CNT, and centrifuged again after beating for 30 minutes. The filter cake was dried at 110°C for 6 hours, pure N 2 Calcined at 360°C for 4 hours in the atmosphere, the stoichiometric formula is Pd 0.016 Zn 1 - 9.6% CNT catalyst (oxidation precursor state), pressed i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com