Isoquinolinium compound, pharmaceutical composition containing the same, preparation and use thereof
A compound and isoquinoline technology, applied in the field of isoquinoline compounds or their salts, can solve the problems of increased myocardial oxygen consumption, accelerated heart rate and the like, and achieve the effects of enhanced myocardial contractility and good anti-arrhythmic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
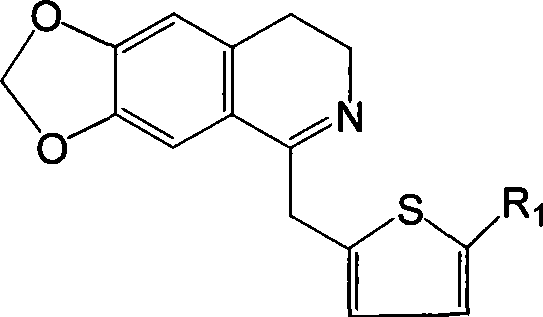
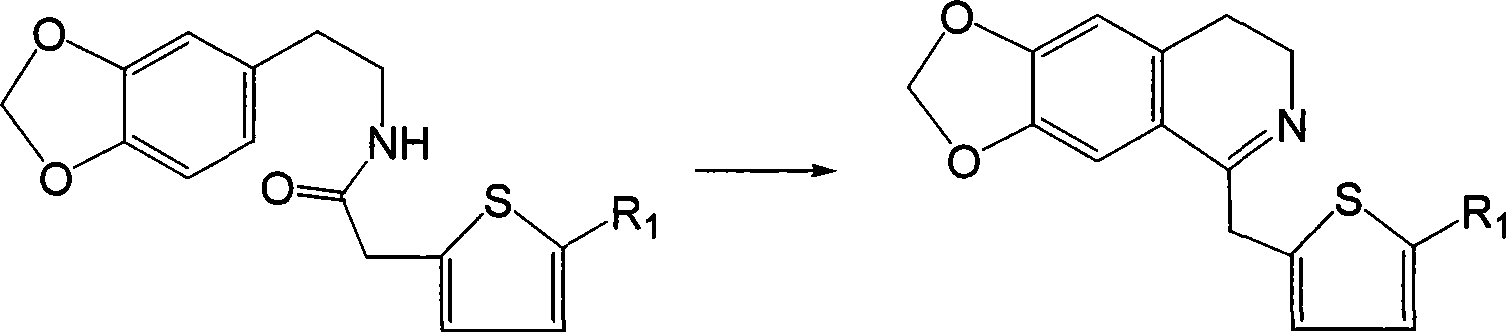
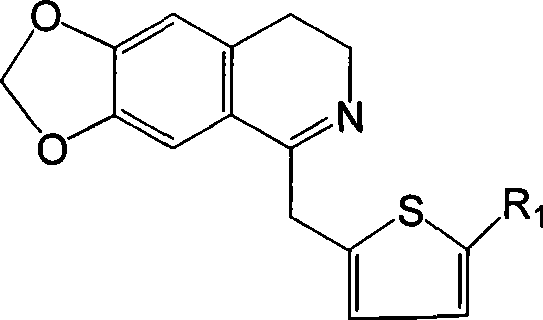
[0042] Example 1 6,7-methylenedioxy-1-(2-thienylmethyl)-3,4-dihydroisoquinoline
[0043] Dissolve 2.75g (10mmol) N-2-thiopheneacetyl-3,4-methylenedioxyphenethylamine in 60ml chloroform, add 2ml (20mmol) POCl dropwise 3 , after the addition, heat to reflux until the point plate reaction shows that the reactant is completely consumed (about 3 hours), and the excess POCl is evaporated under reduced pressure. 3 and chloroform, the residue was washed with 1:1 benzene-hexane, solidified, and the crude product was collected by filtration, recrystallized from methanol-ether (volume ratio 1:1) to obtain 6,7-methylenedioxy-1-(2 -2.89 g of -thienylmethyl)-3,4-dihydroisoquinoline, yield 98.8%.
Embodiment 2
[0044] Example 2 6,7-methylenedioxy-1-(2-thienylmethyl)-3,4-dihydroisoquinoline hydrochloride
[0045] Dissolve 2.75g (10mmol) N-2-thiopheneacetyl-3,4-methylenedioxyphenethylamine in 60ml chloroform, add 1ml (10mmol) POCl dropwise 3 , the addition is completed, heated to 50 ° C, until the point plate reaction shows that the reactants are completely consumed (about 3 hours), and the excess POCl is evaporated under reduced pressure 3 and chloroform, the residue was washed with 1:1 benzene-hexane, solidified, and the crude product was collected by filtration, recrystallized from methanol-ether (volume ratio 1:1), collected crystals, dissolved in ethanol, and dripped into a saturated ethanol solution of HCl to pH=1, 2.0 g of 6,7-methylenedioxy-1-(2-thienylmethyl)-3,4-dihydroisoquinoline hydrochloride was obtained, the yield was 68%, and the melting point was 201.8-203.0°C .
[0046] Elemental Analysis: C 15 h 14 cNO 2 S: Calculated %: C 58.53, H 4.58, N 4.55, Cl 11.52
[004...
Embodiment 3
[0049] Example 3 6,7-methylenedioxy-1-(2-thienylmethyl)-3,4-dihydroisoquinoline tartrate
[0050] Dissolve 2.75g (10mmol) N-2-thiopheneacetyl-3,4-methylenedioxyphenethylamine in 60ml benzene, add 1.5ml (15mmol) POCl dropwise 3 , the addition is complete, heated to 80 ° C, the reactant disappears (about 3 hours), and the excess POCl is evaporated under reduced pressure 3 and chloroform, the residue was washed with 1:1 benzene-hexane, solidified, and the crude product was collected by filtration, recrystallized from methanol-ether (volume ratio 1:1), and the crystals were collected to obtain 6,7-methylenedichloro-1 -(2-thienylmethyl)-3,4-dihydroisoquinoline, dissolved in ethyl acetate, dropwise add saturated 8wt% tartaric acid ethyl acetate solution until pH=1.5, stir to precipitate crystals, filter , The filter cake was washed with ethyl acetate and dried to give 6,7-methylenedichloro-1-(2-thienylmethyl)-3,4-dihydroisoquinoline tartrate.
[0051] Reference Example 2 N-2--5'-m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com