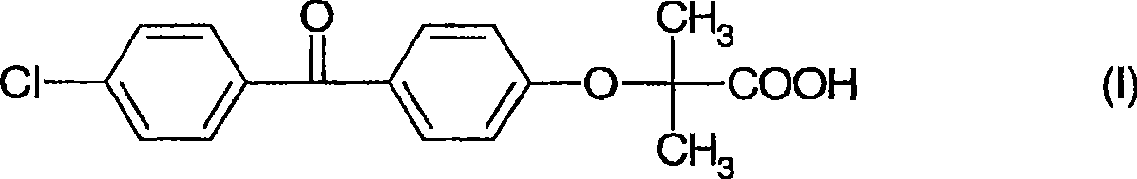
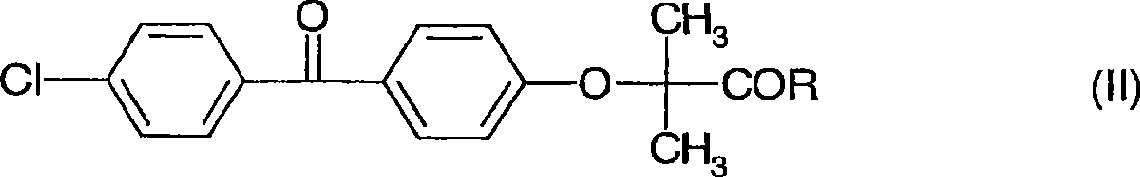
Formulation comprising fenofibric acid, a physiologically acceptable salt or derivative thereof
A fenofibric acid and preparation technology, applied in the field of oral administration of fenofibric acid to its physiologically acceptable salt or derivatives, can solve problems such as recrystallization and reduction of drug bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] Fenofibrate (120g, equivalent to 15% w / w), HP 55 S (hydroxypropylmethylcellulose phthalate, ShinEtsu, 672g, equivalent to 84% w / w) and colloidal Silica (Aerosil 200, 8 g, corresponding to 1% w / w) was mixed in a turbulent mixer for 4 minutes. The powder mixture was then extruded in a twin-screw extruder (screw diameter 18 mm) at a feed rate of 1.0 kg / h at a melt temperature of 165°C. A clear, transparent rope-like melt with a thickness of approximately 1.0 cm was extruded. The material is directly formed into tablets (oval shape) by calendering between two co-rotating rollers. High hardness clear, transparent tablets with a tablet weight of about 550 mg are obtained by this method.
Embodiment 2
[0145] The tablets of Example 1 were milled in a laboratory mill and the resulting powder was analyzed by DSC between 20 and 250° C. (Mettler Toledo DSC-820; 8.45 mg in closed pan, 10 K / min). No endothermic melting peak was observed, indicating that fenofibrate exists in the polymer backbone in an amorphous form.
Embodiment 3
[0147] The powder obtained by grinding the tablet of Example 2 was subjected to WAXS (Wide Angle X-ray Scattering; Bruker AXS D-5005) analysis. No distinct peaks were seen in WAXS, indicating the absence of crystalline fenofibrate in this formulation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com