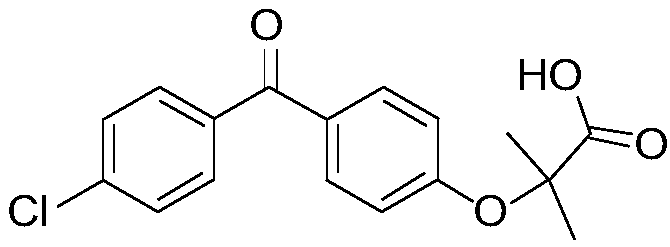
Stable fenofibric acid tablet and preparation method thereof
A fenofibric acid and stable technology, applied in the field of medicine, can solve the problems of complex preparation process and high cost, and achieve the effects of simplified preparation process, rapid disintegration and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A kind of stable fenofibric acid tablet in the present embodiment is made up of the component of following weight ratio:
[0025] components
weight ratio
Raw material: fenofibric acid
1
Filler: microcrystalline cellulose
6.25
Disintegrant: Crospovidone
0.43
Binder: Copovidone
0.4
0.07
[0026] The preparation method of fenofibric acid tablet in the above-mentioned embodiment, described method is powder direct compression technology, specifically is made up of the following steps:
[0027] 1) Pass the fenofibric acid raw material and auxiliary materials through a 60-200-mesh sieve for later use;
[0028] 2) Weigh the material according to the weight ratio of fenofibric acid, microcrystalline cellulose, crospovidone, copovidone and magnesium stearate to 1:6.25:0.43:0.4:0.07; Norbenic acid, microcrystalline cellulose, crospovidone and copovidone are mixed evenly;
[0029] 3)...
Embodiment 2
[0031] A kind of stable fenofibric acid tablet in the present embodiment is made up of the component of following weight ratio:
[0032] components
Proportion
Raw material: fenofibric acid
1
Filler: microcrystalline cellulose
4
Disintegrant: Crospovidone
2
Binder: Copovidone
0.1
0.01
[0033] The preparation method of fenofibric acid tablet in the above-mentioned embodiment, described method is powder direct compression technology, specifically is made up of the following steps:
[0034] 1) Pass the fenofibric acid raw material and auxiliary materials through a 60-200-mesh sieve for later use;
[0035] 2) Weigh the material according to the weight ratio of fenofibric acid, microcrystalline cellulose, crospovidone, copovidone and magnesium stearate at 1:4:2:0.1:0.01; Norbenic acid, microcrystalline cellulose, crospovidone and copovidone are mixed evenly;
[0036] 3) Adding magnes...
Embodiment 3
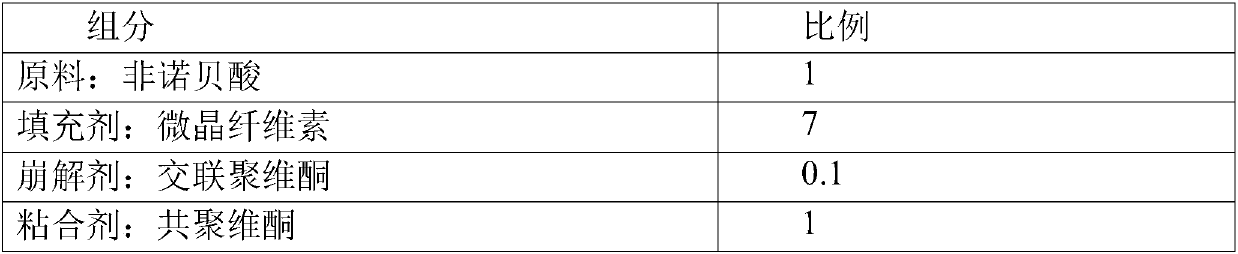
[0038] A kind of stable fenofibric acid tablet in the present embodiment is made up of the component of following weight ratio:
[0039]
[0040]
[0041] The preparation method of fenofibric acid tablet in the above-mentioned embodiment, described method is powder direct compression technology, specifically is made up of the following steps:
[0042] 1) Pass the fenofibric acid raw material and auxiliary materials through a 60-200-mesh sieve for later use;
[0043] 2) Weigh the material according to the weight ratio of fenofibric acid, microcrystalline cellulose, crospovidone, copovidone and magnesium stearate at 1:7:0.1:1:0.1; Norbenic acid, microcrystalline cellulose, crospovidone and copovidone are mixed evenly;
[0044] 3) Adding magnesium stearate to the mixture obtained in step 2), mixing evenly, and pressing into tablets to obtain the fenofibric acid tablets, the hardness of the obtained fenofibric acid tablets is 5-9kgf.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com