Novel PRRS virus receptor and block inhibitor thereof
A technology of receptor blocker, virus, applied in the field of biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Discovery and identification of novel PRRSV cell receptors
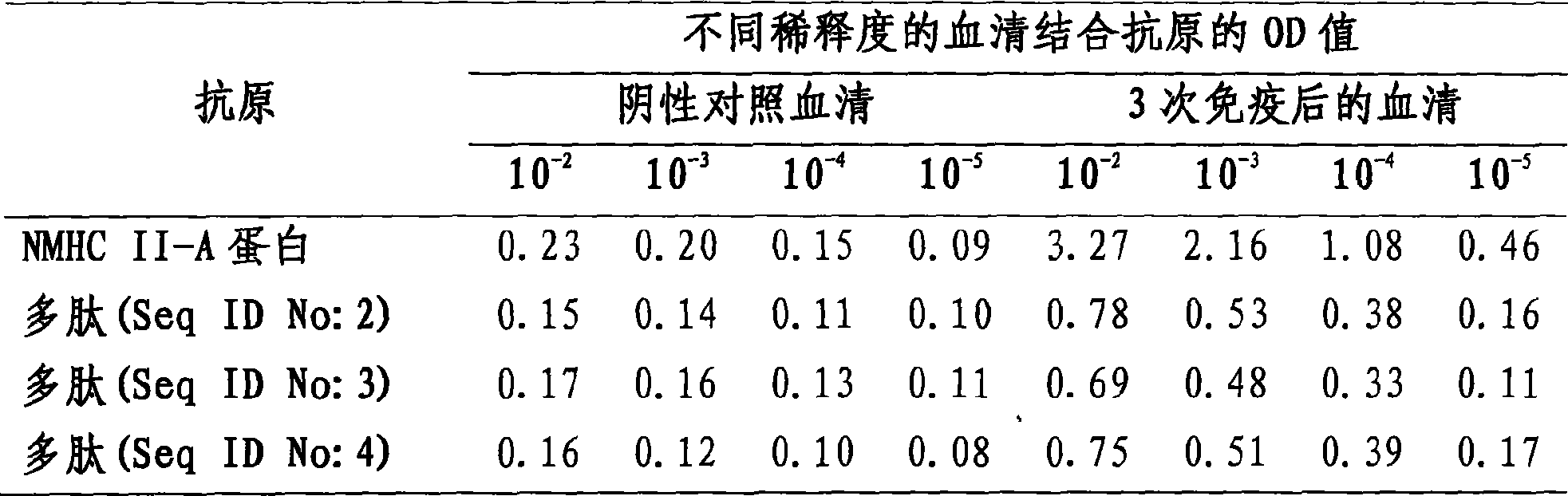
[0019] A soluble protein was purified from MA-104 and PAM cells using a monoclonal antibody idiotype antibody that mimics PRRSV GP5 membrane antigen (Zhou EM, Xiao YH, Shi YF, et al.J.Virol.Methods, 2008, 149: 300 -308): 50 μg of soluble protein was separated by 7.5% SDS-PAGE, and the separated protein was hydrolyzed by pepsin, and the hydrolyzed fragment was separated by microcapillary high-pressure liquid chromatography, and the separated fragment was ionized by nano-electrospray, and analyzed by ion trapping mass spectrometry instrument analysis. The obtained hydrolyzed fragment sequence was compared with the GenBank amino acid sequence library, and the receptor protein was non-muscle myosin type II reconnected A (NMHCII-A).
Embodiment 2
[0020] Embodiment 2 NMHC II-A artificial polypeptide synthesis
[0021] The polypeptide sequence formulated according to the NMHC II-A reconnected carboxy-terminal sequence is synthesized by the solid-phase peptide synthesis method. The specific method is:
[0022] Mix and shake 2-Chlorotrityl Chloride Resin resin and DMF for 30-60 minutes to swell the resin; filter out DMF through sand core, add 3 times molar excess of the first amino acid at the C-terminal to connect with the resin, and then add 10 times molar excess of DIEA , finally add DMF to dissolve, shake for 30-60min; remove DMF, add 20% piperidine DMF solution, react for 5-15min; take more than a dozen resins, wash with ethanol three times, add ninhydrin, KCN, phenol solution each drop, Heating at 105°C-110°C for 5min to detect the reactants. Wash twice with DMF, methanol and DMF sequentially. Condensate the ligation product by one of the following methods.
[0023] Method a: Add a three-fold excess of protected a...
Embodiment 4
[0031] Example 4 Preparation of Anti-NMHC II-A Protein and Artificial Polypeptide Antibody
[0032] Protein and polypeptide-aluminum adjuvant preparation: NMHC II-A protein or polypeptide-KLH coupling product was dissolved in 0.005MPBS, pH 6.2 (final volume 10mL), slowly added potassium aluminum sulfate dissolved in 0.005M PBS (pH 6.2) ( The ratio is 50μg protein / 0.4mg aluminum), the pH is raised to 6.8-7.3, stirred for 2 hours, centrifuged at 800g for 10 minutes, the protein-aluminum adjuvant precipitate is washed once with normal saline, and dissolved in 0.1M PBS. Proteins and peptides can also be used with other adjuvants, such as Freund's adjuvant.
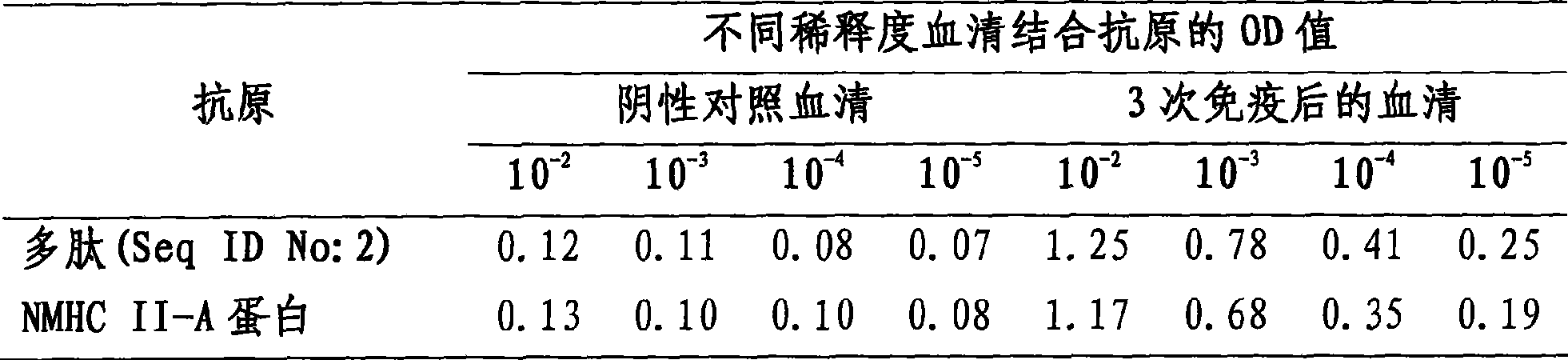
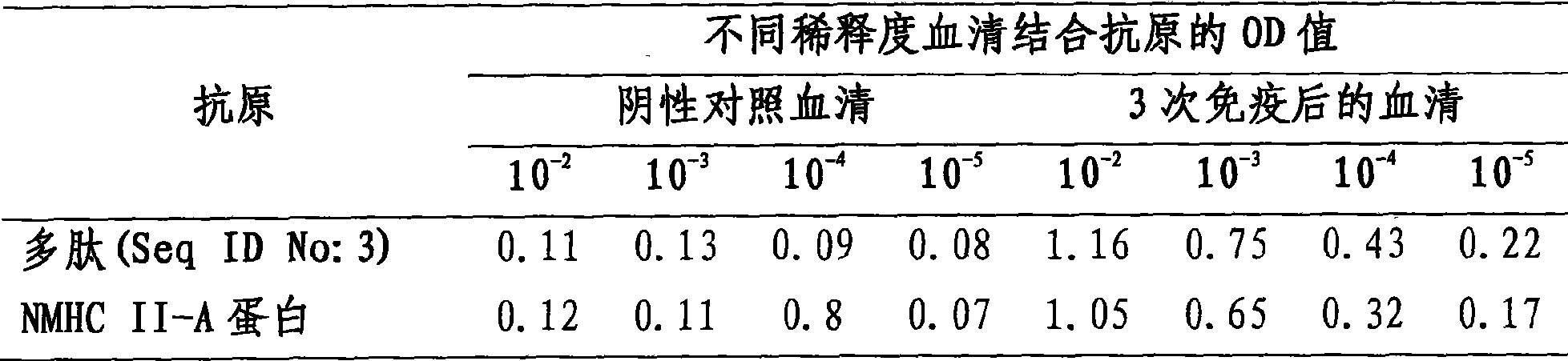
[0033] Immunization procedure: each mouse was immunized intraperitoneally with 50 μg of NMHC II-A protein or artificial polypeptide for a total of three times, and the serum of the mice was collected to detect and identify antibodies. Antibodies against NMHC II-A protein or artificial polypeptide can also be used in other ani...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com