Positively charged water-soluble prodrugs of aryl- and heteroarylpropionic acids with very fast skin penetration rate
An aryl and alkyl technology, applied in the field of positively charged water-soluble aryl and heteroaryl propionic acid prodrugs with fast skin penetration speed, can solve the problem of slow skin penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
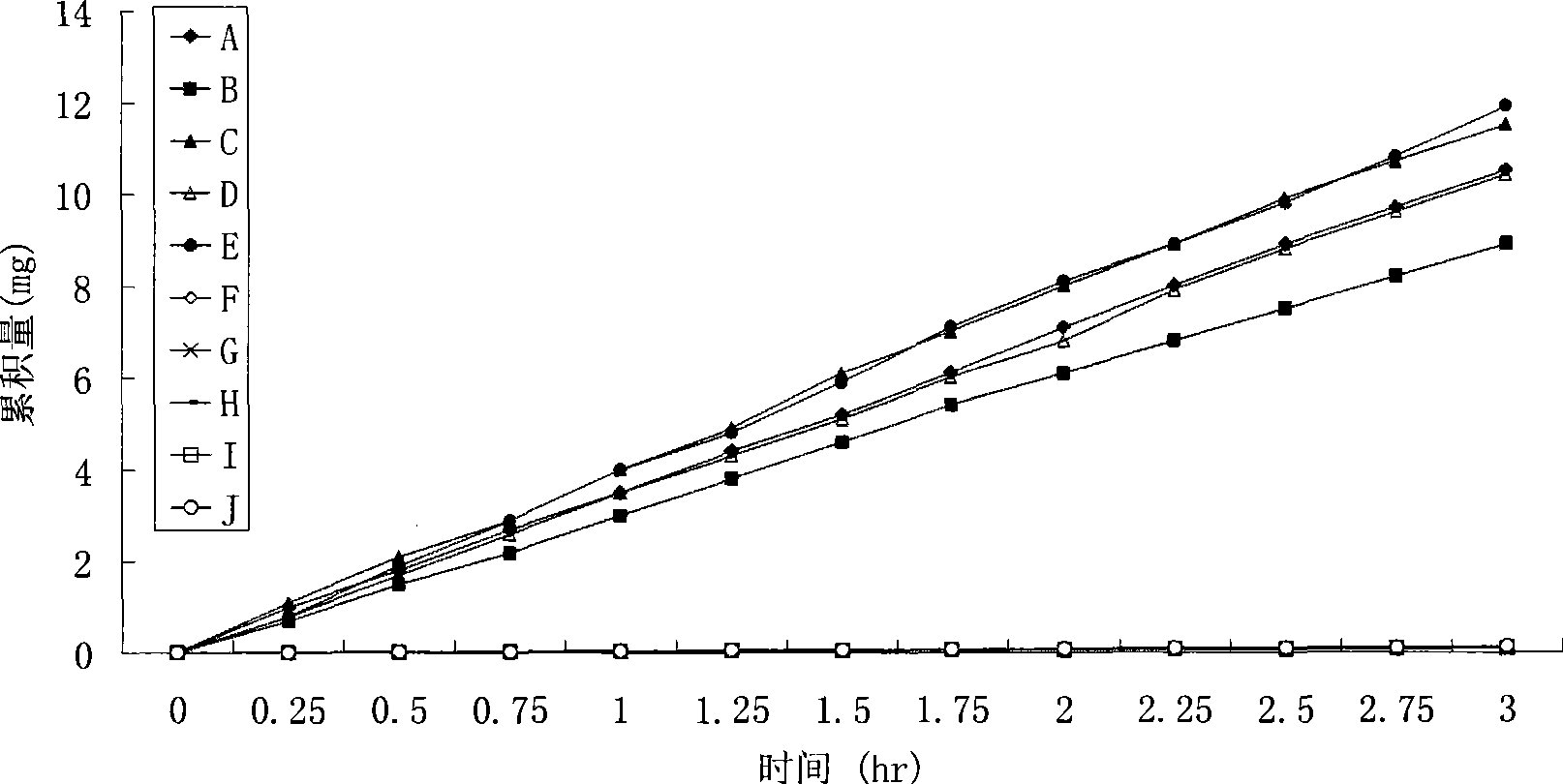
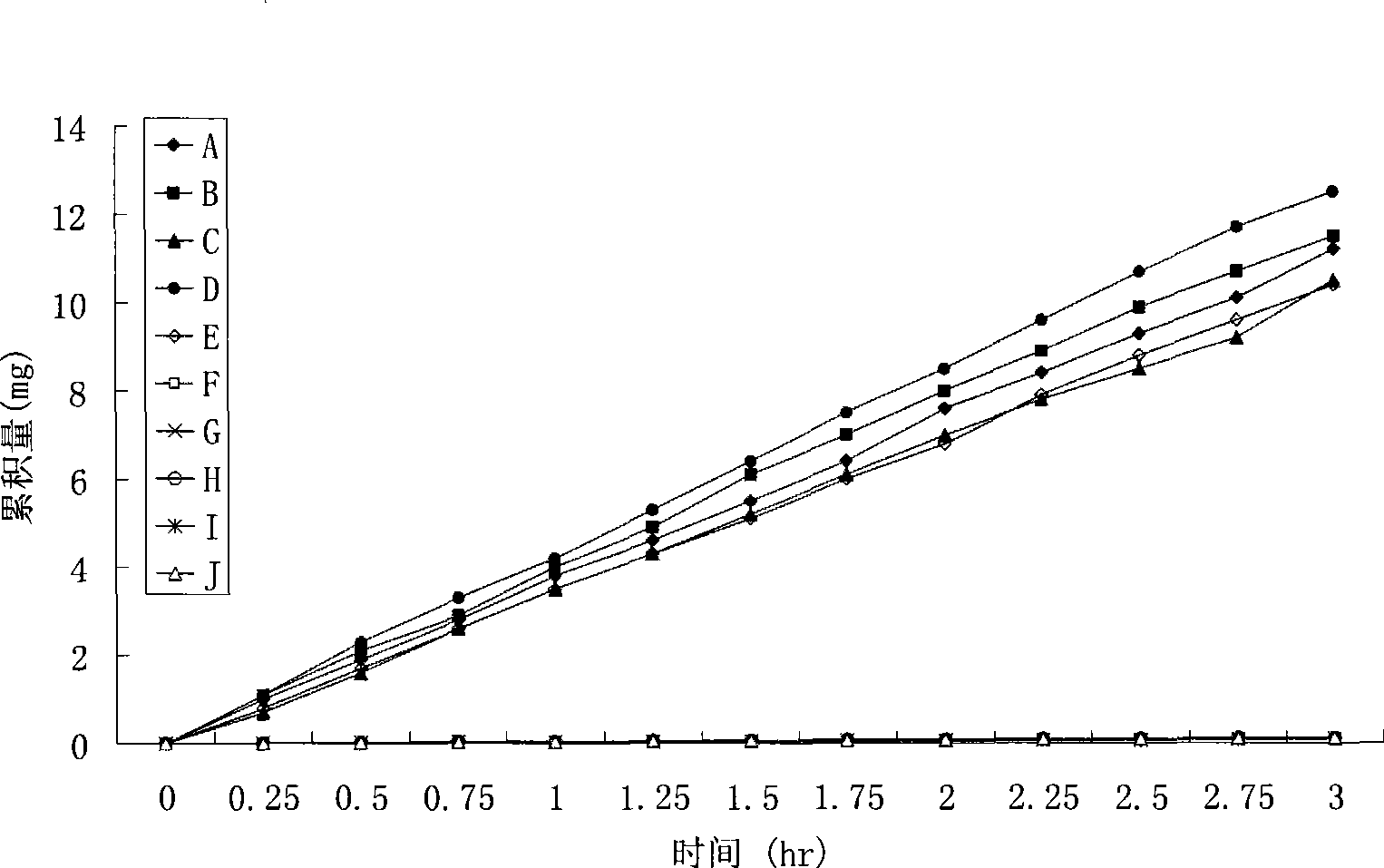
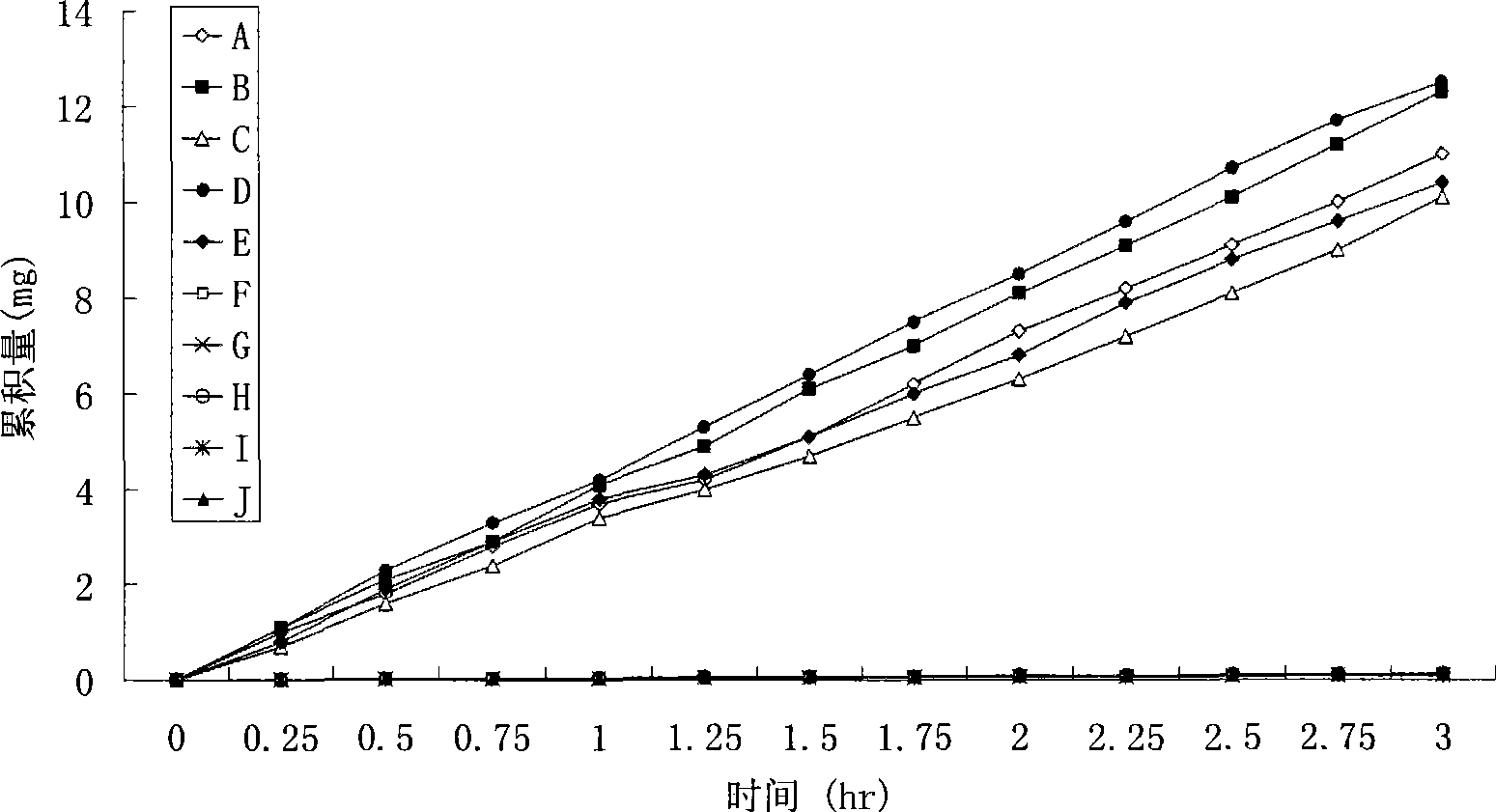
Image
Examples
Embodiment approach
[0105] Synthesis of Diethylaminoethyl α-Methyl-4-(2-thienyl)phenylacetate Acetate
[0106] 28.1 g (0.1 mol) of α-methyl-4-(2-thienoyl)phenylacetyl chloride was dissolved in 100 ml of chloroform and cooled to 0°C. 15 ml of triethylamine and 11.7 g (0.1 mol) of diethylaminoethanol were added to the reaction solution. Stir at room temperature for 3 hours. The solvent was evaporated to dryness. The solid mixture evaporated to dryness was suspended in 300 ml of methanol, and 200 ml of 5% aqueous sodium bicarbonate solution was added with stirring, followed by stirring at room temperature for 3 hours. The mixture was evaporated to dryness. To the evaporated mixture was added 300 ml of methanol for dissolution. The solid was removed by filtration and washed with methanol. The solution was evaporated to dryness, and 200ml of chloroform was added to dissolve it. 6 g of acetic acid was added to the reaction solution with stirring. A small amount of solid formed was removed by fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com