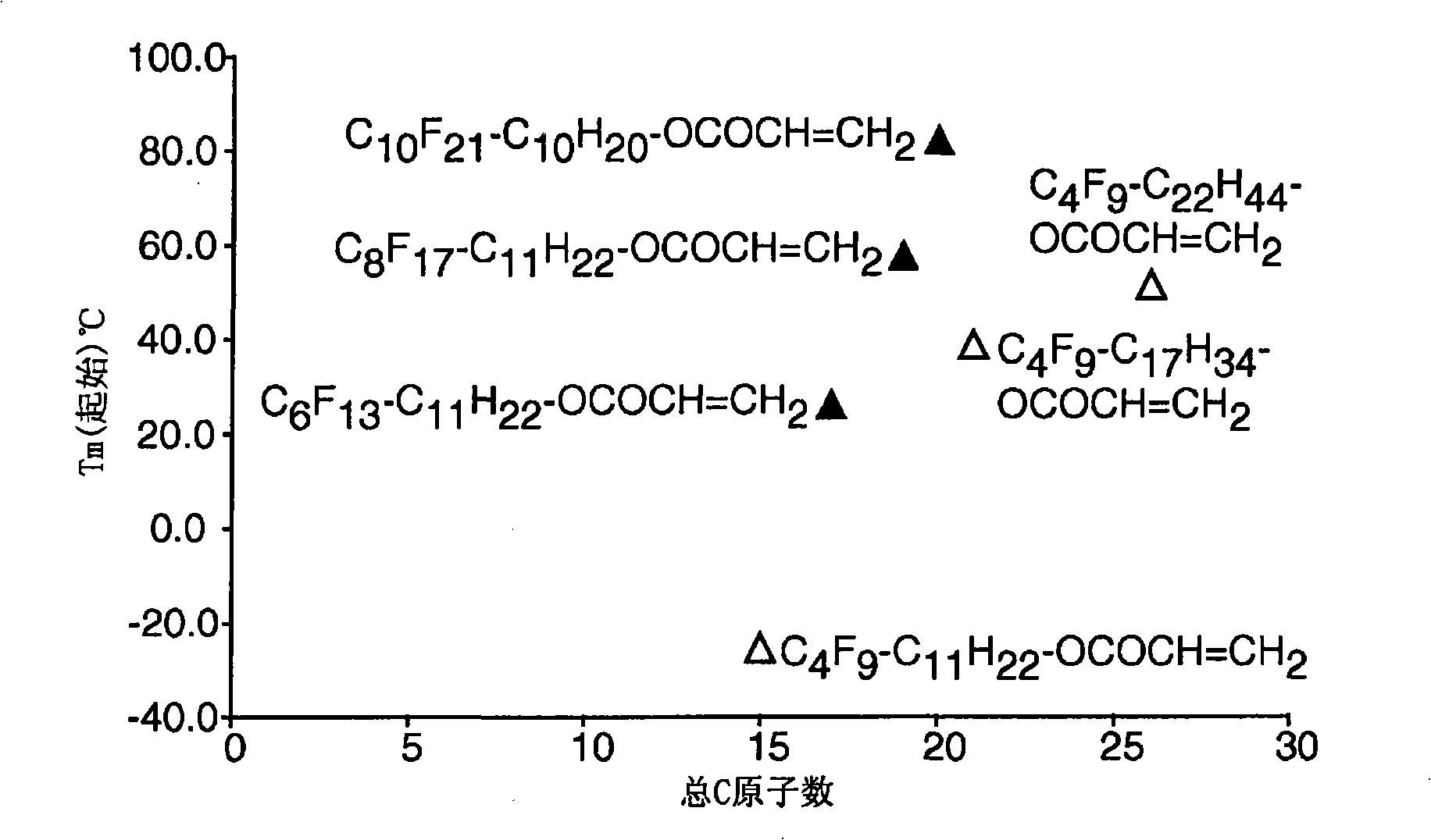
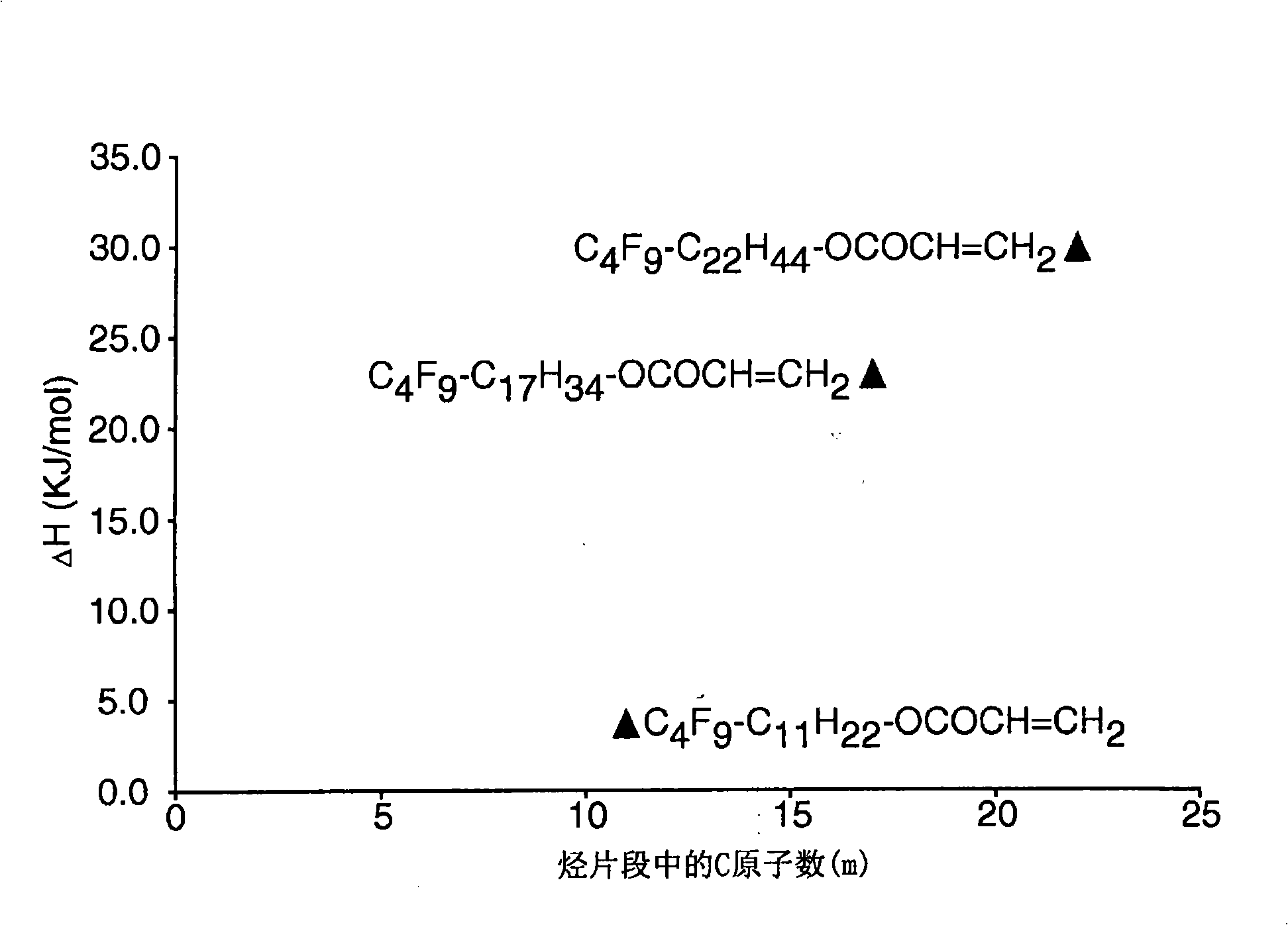
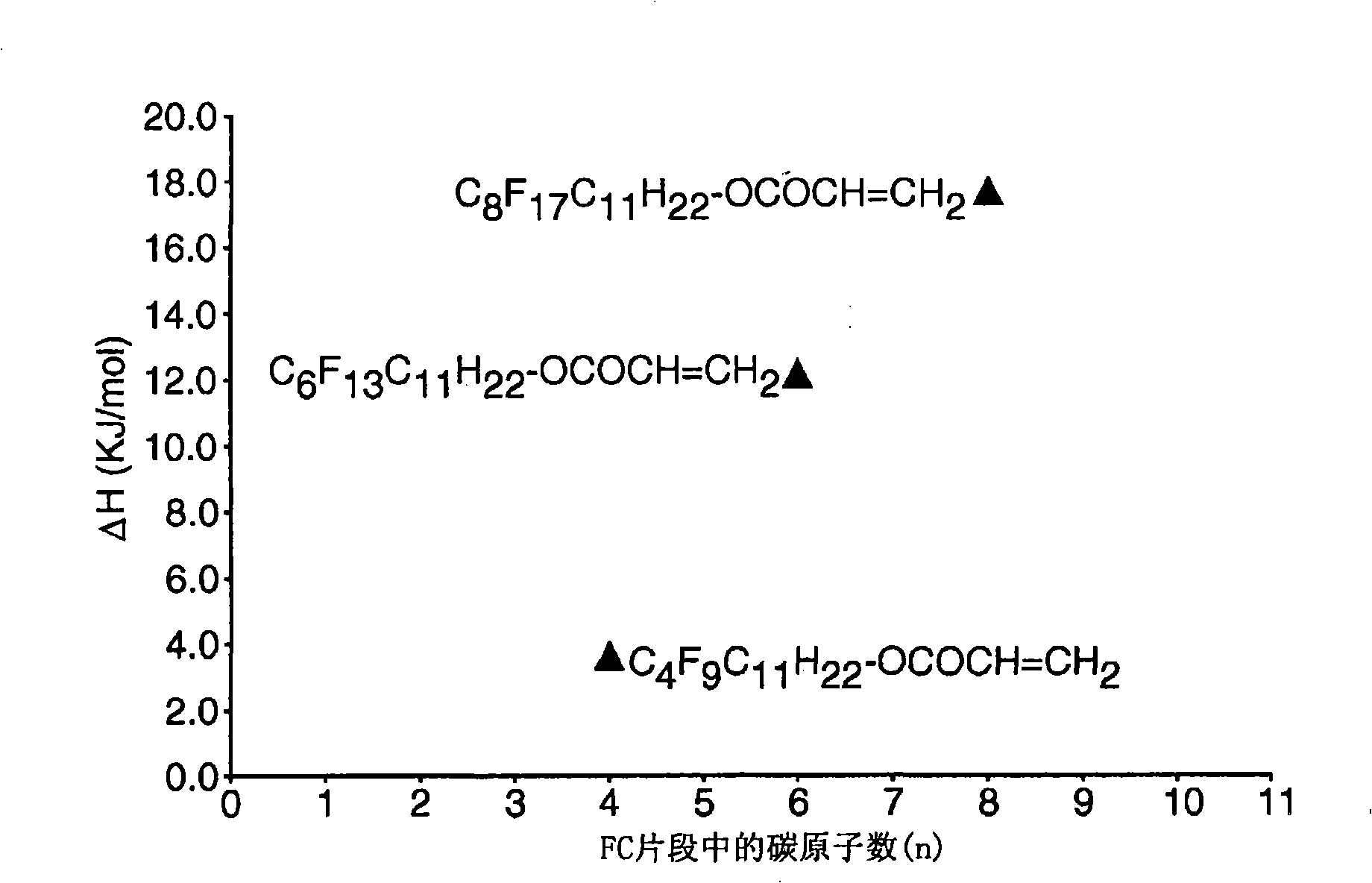Side chain fluorochemicals with crystallizable spacer groups
A compound and group technology, applied in the field of fluorine-containing compounds, to achieve the effect of easy processing and handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0067] All chemicals used in the following examples were or could be obtained from Sigma-Aldrich, St. Louis, MO unless otherwise indicated.
example 1
[0069] CH 2 =CH(CH 2 ) m Synthesis of OH, m=15 and 20
[0070] CH was prepared essentially as described in the multistep synthesis of S. Mirviss, J. Org. Chem. 54 1948 (1989) 2 =CH(CH 2 ) 14 COOH and CH 2 =CH(CH 2 ) 19 COOH.
[0071] Under nitrogen atmosphere, 21.9g 0.082mol CH 2 =CH(CH 2 ) 14 A mixture of COOH and 250 ml of anhydrous diethyl ether was added dropwise to 100 ml of a 1M solution of lithium aluminum hydride in stirred diethyl ether in a dry 500 ml round bottom flask. After 30 minutes of adding the mixture to the flask, the exothermic reaction was allowed to raise the temperature of the reaction mixture to reflux temperature. The reaction was allowed to continue to reflux for four hours, then cooled to 7°C with an external ice bath.
[0072] The cooled reaction mixture was then slowly hydrolyzed using 4 mL of water, 4 mL of 15% sodium hydroxide, and 12 mL of water delivered by syringe through the septum port of a 500 mL round bottom flask...
example 2
[0085] C 4 F 9 (CH 2 ) 22 Synthesis of OH
[0086] to synthesize C with the above 4 f 9 (CH 2 ) 17 In a similar fashion to OH, C was treated with zinc in glacial acetic acid 4 f 9 CH 2 CHI(CH 2 ) 20 OH to provide a mixture of reduction products alcohol and acetic acid and their corresponding alkenes. The mixture was treated with potassium hydroxide essentially as above to hydrolyze ethyl acetate, and finally the mixture was treated with hydrogen to remove olefinic impurities and recrystallized from n-hexane. Approximately 16.8 g of white solid C was obtained from this step 4 f 9 (CH 2 ) 22 OH, 98% pure by GC / MS.
[0087] C 4 F 9 (CH 2 ) 22 Synthesis of OAcr
[0088] Then to synthesize C with the above 4 f 9 (CH 2 ) 17 In a similar fashion to OAcr, the C 4 f 9 (CH 2 ) 22 OH is converted to acrylate. The final acrylate product was about 97% pure as measured by GC / MS. Further analysis of the sample by fast atom bombard...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com