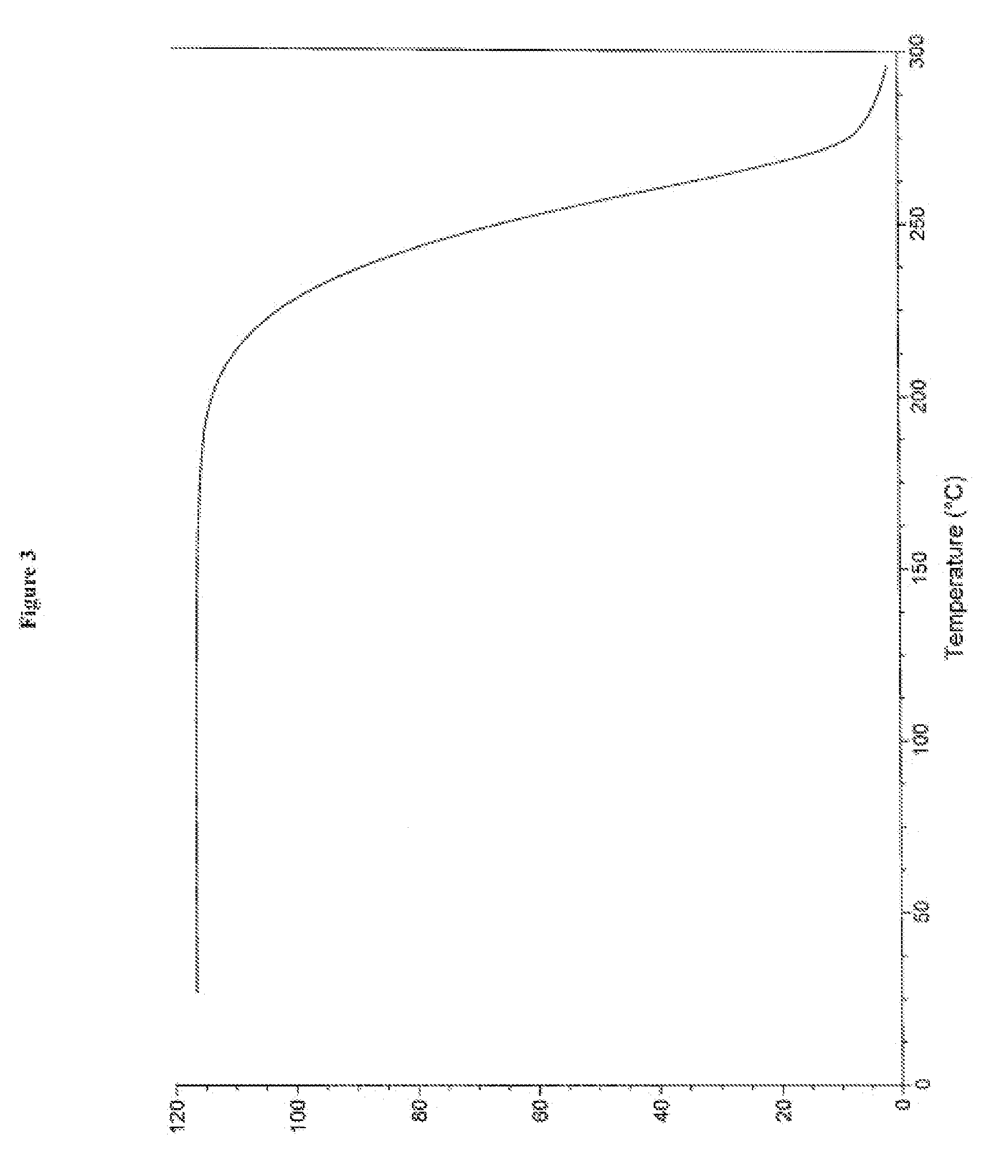
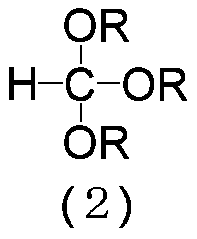
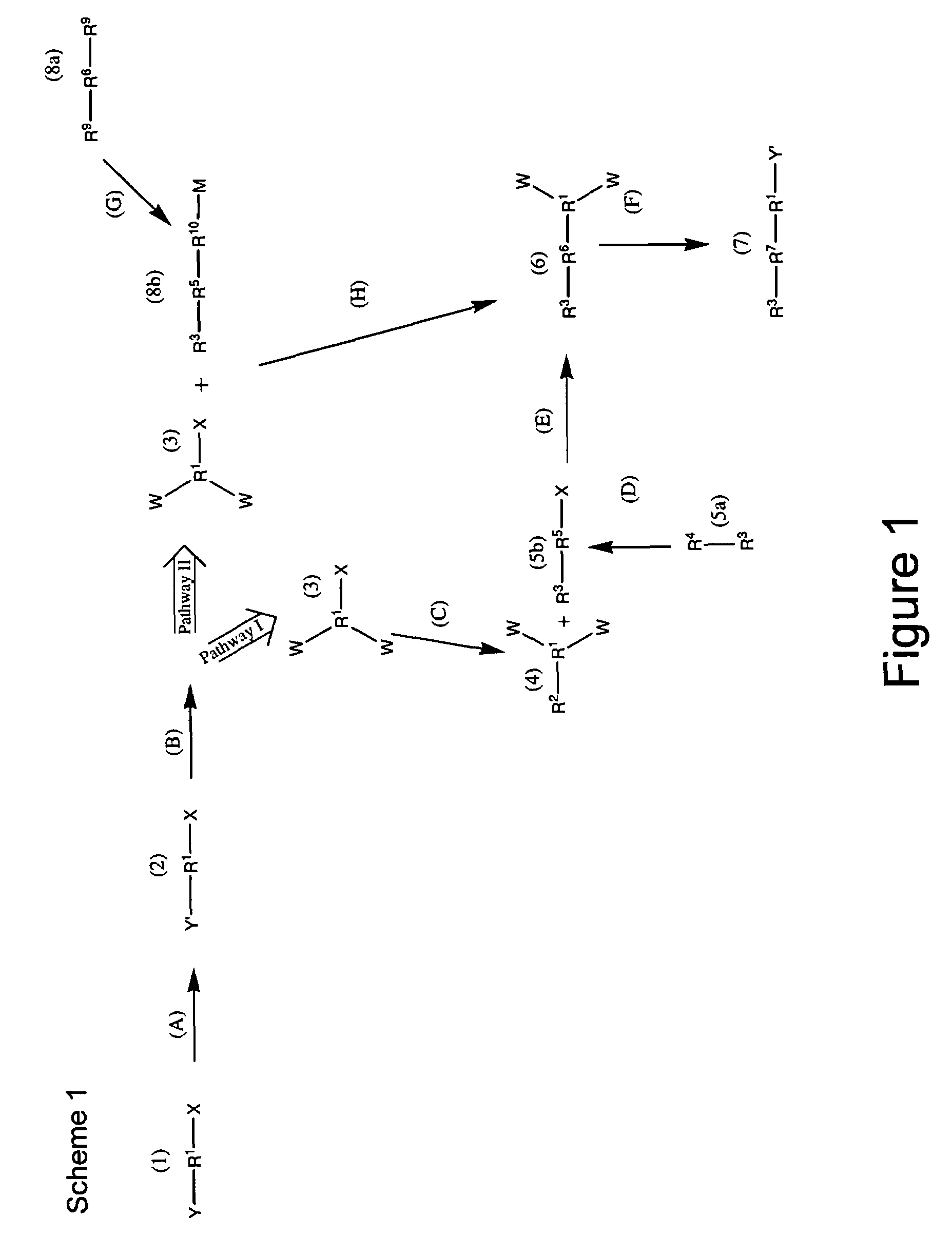
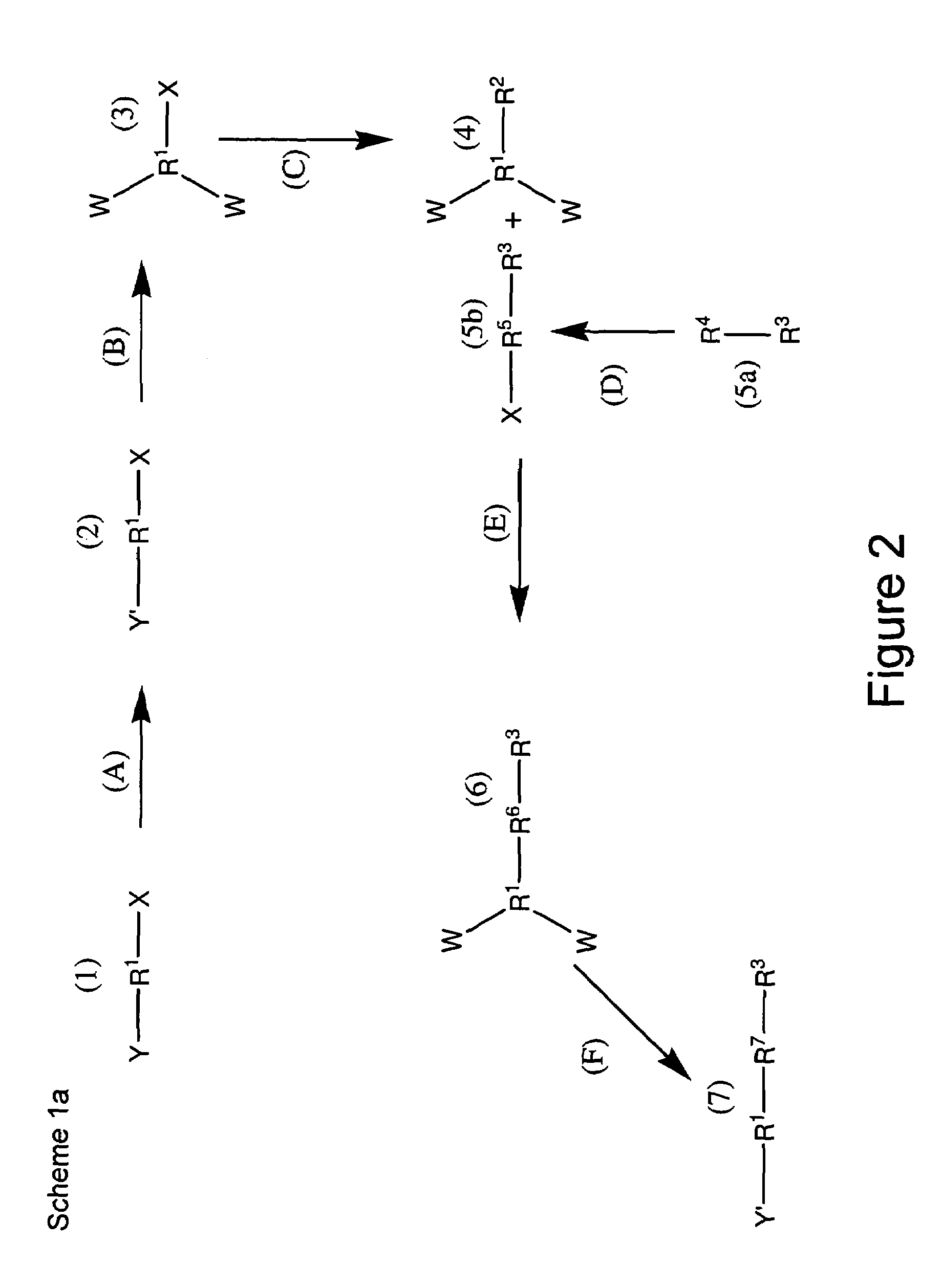
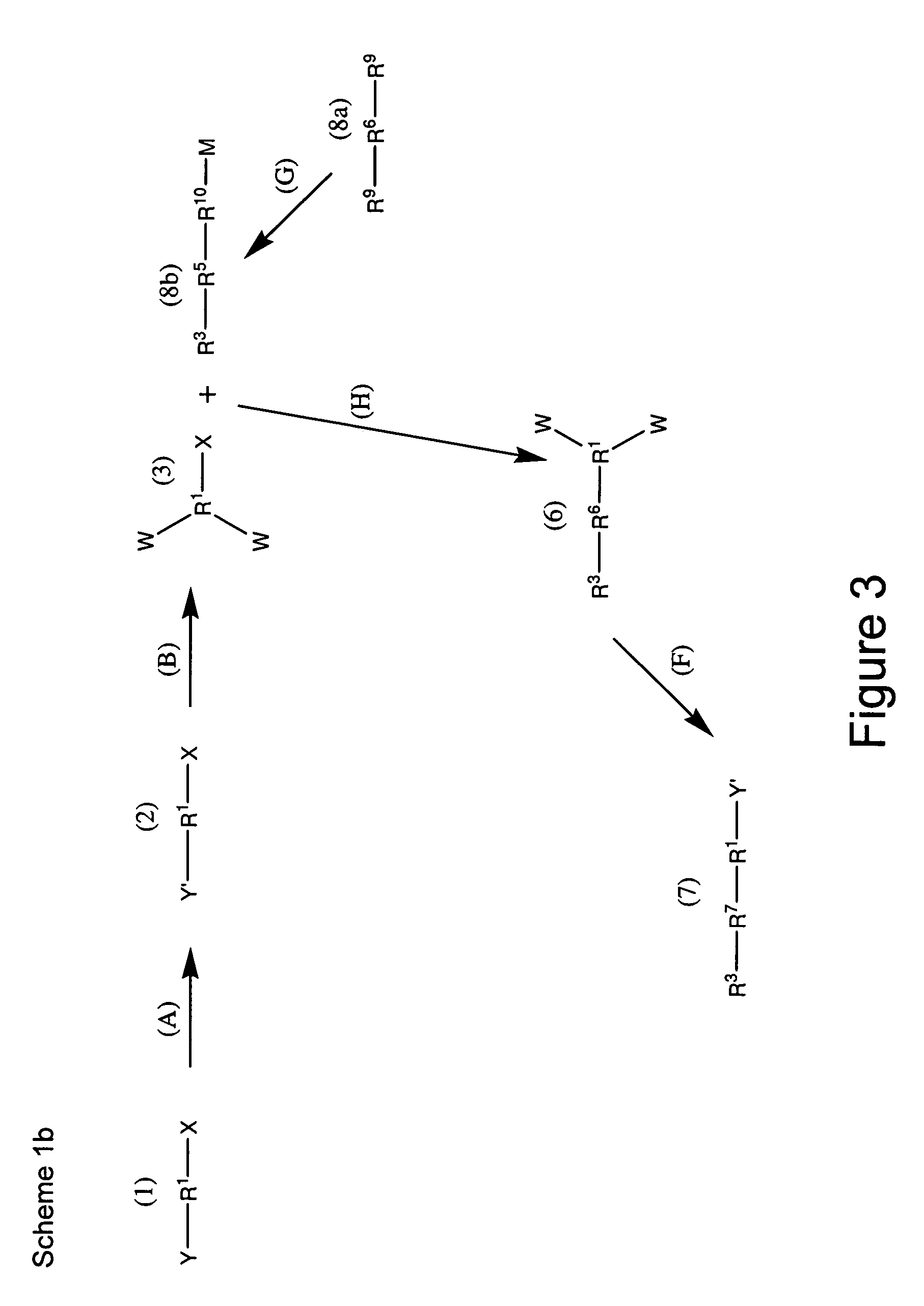
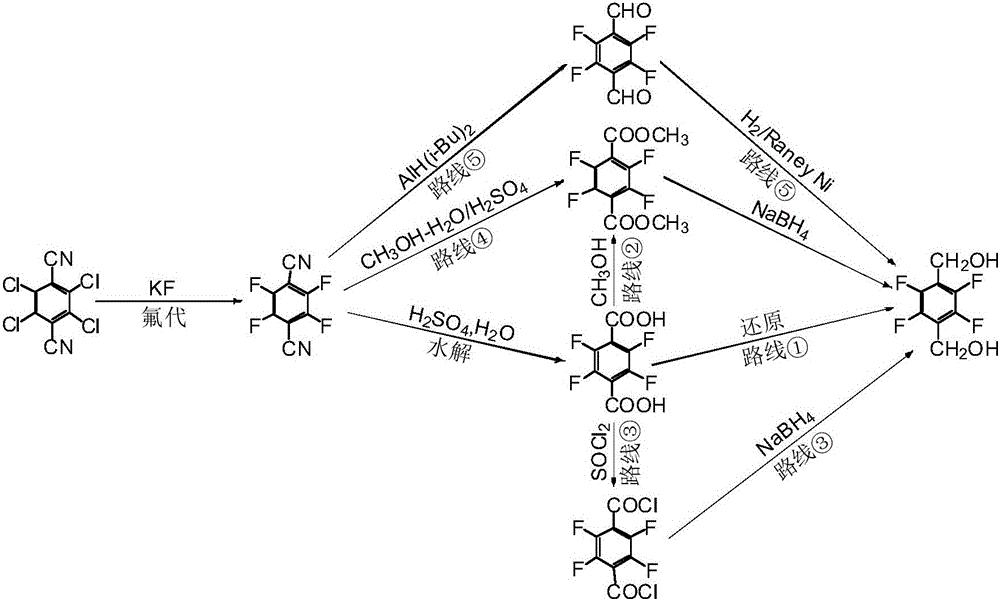
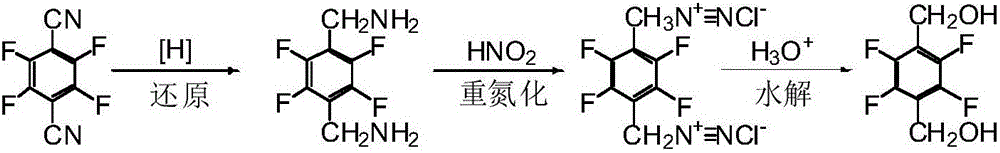
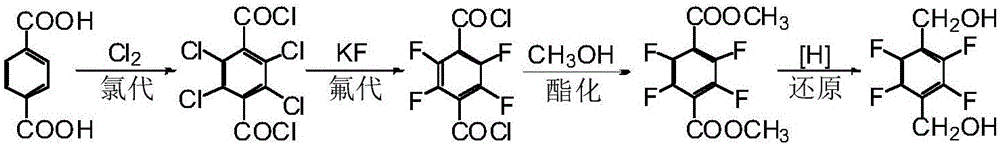
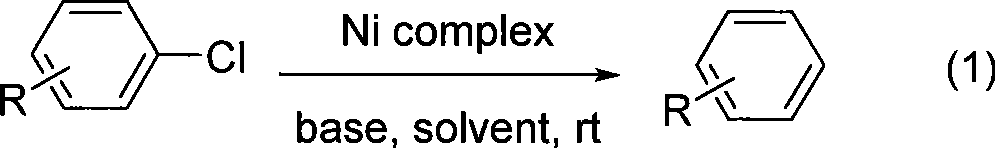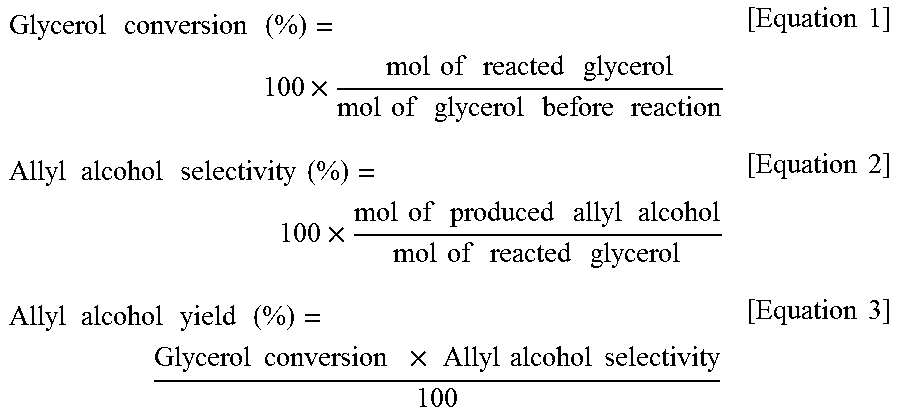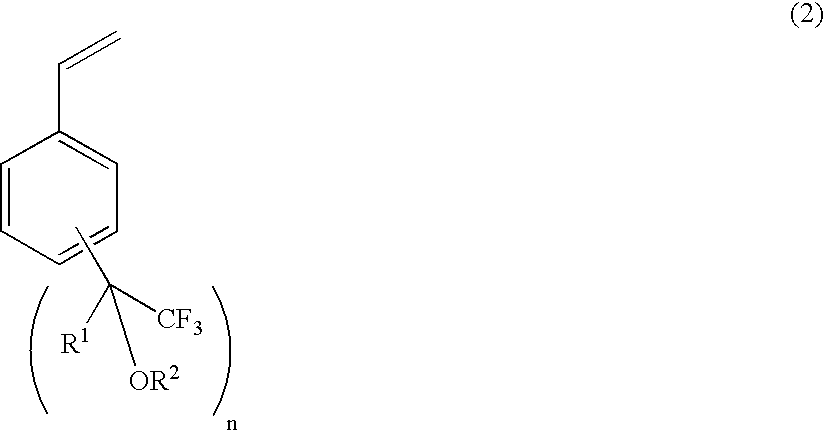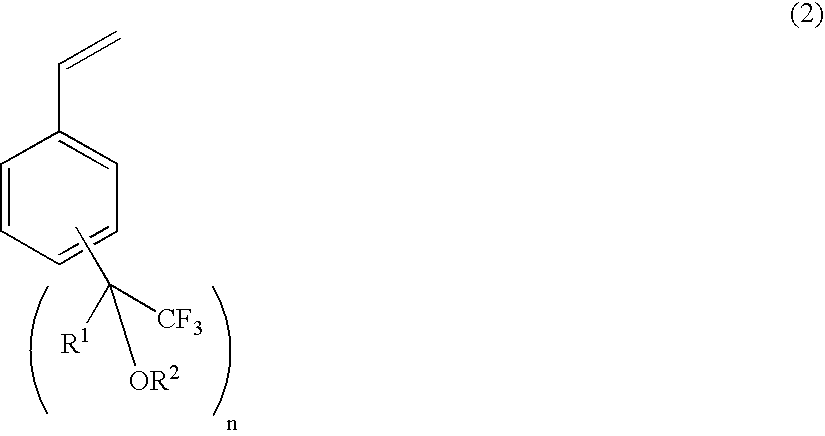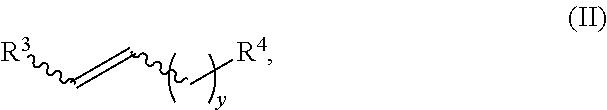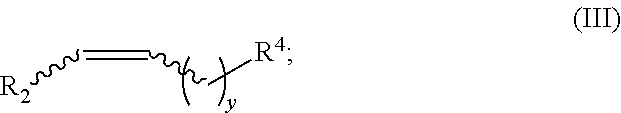Patents
Literature
70results about "Preparation by halogen elimination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
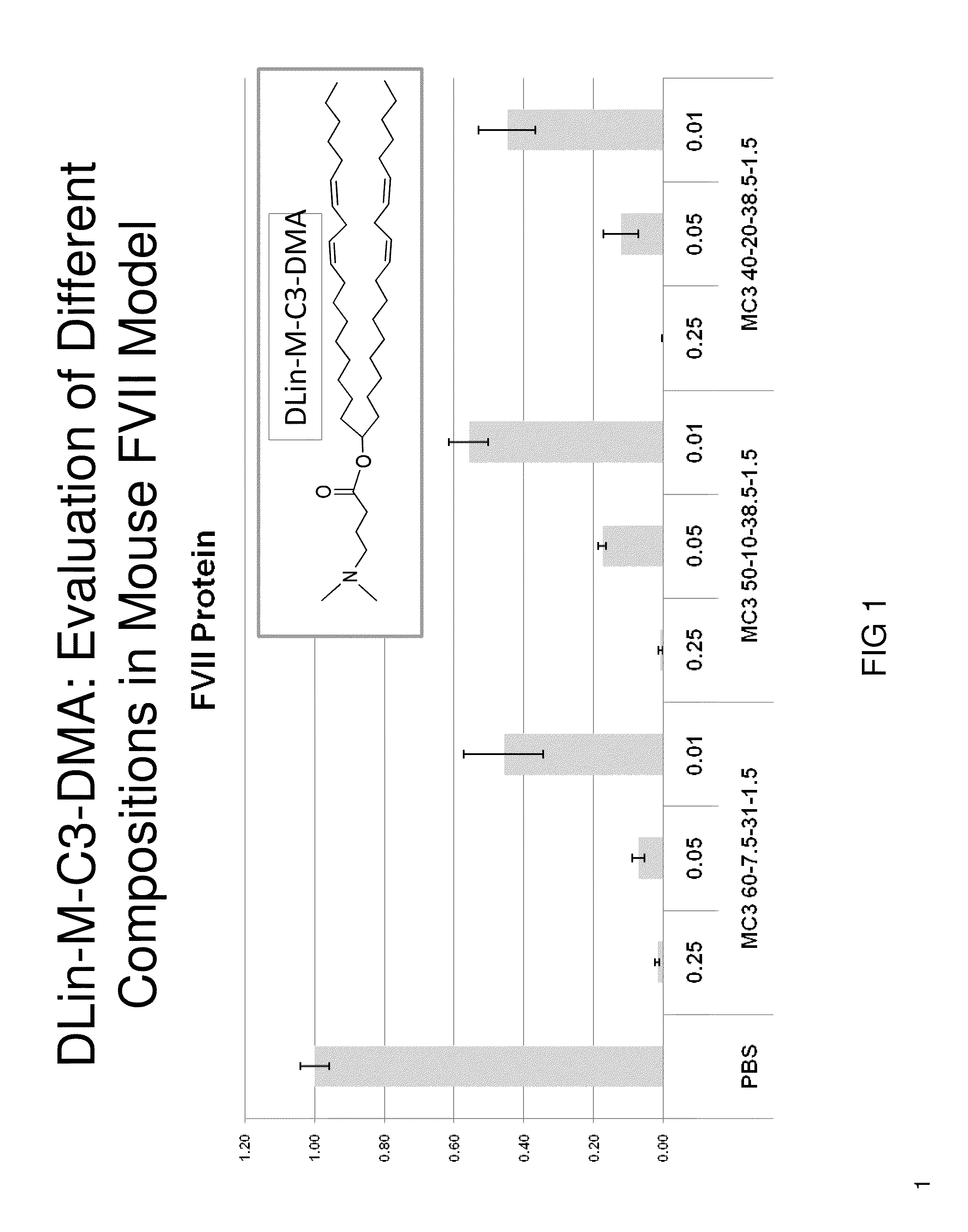
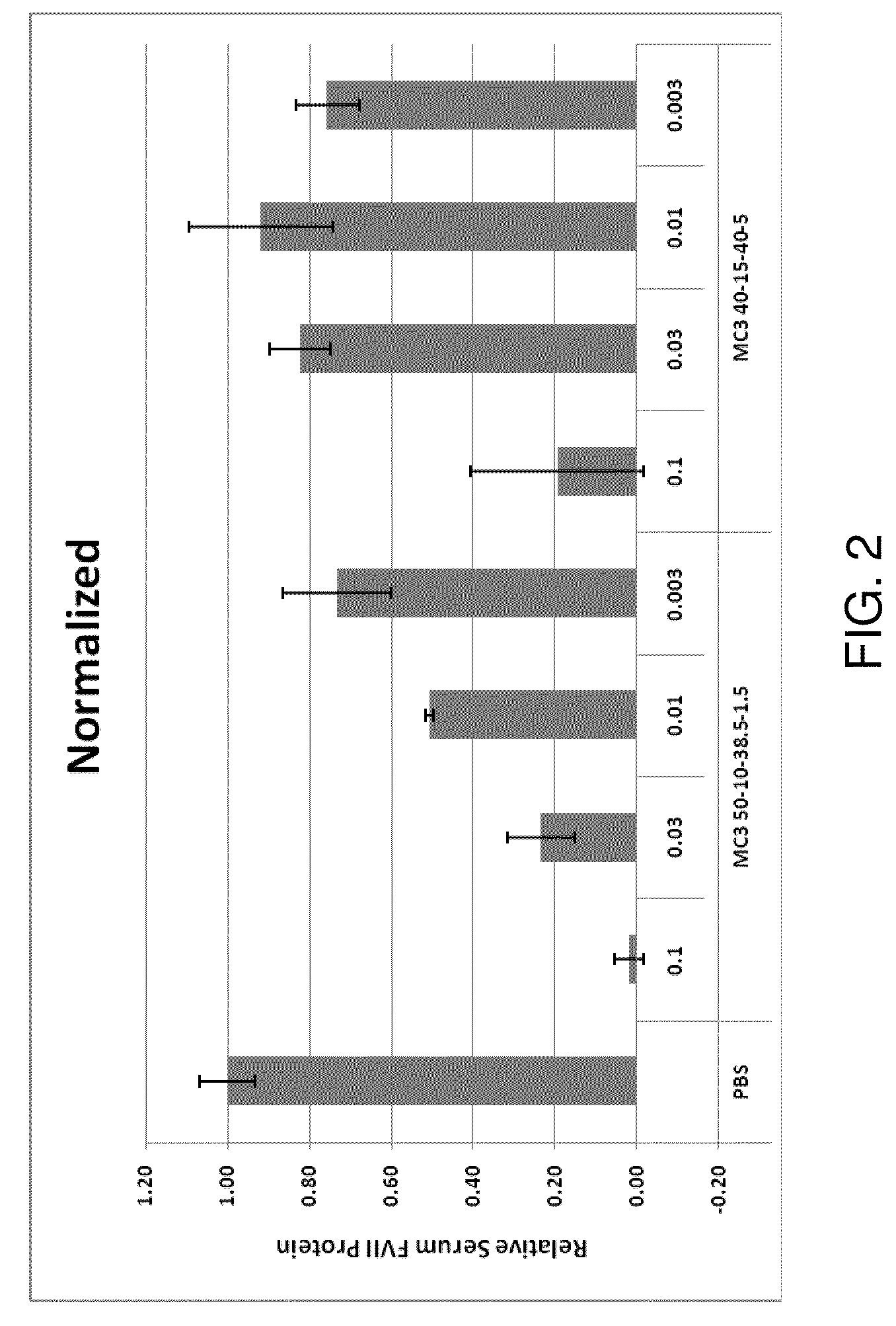
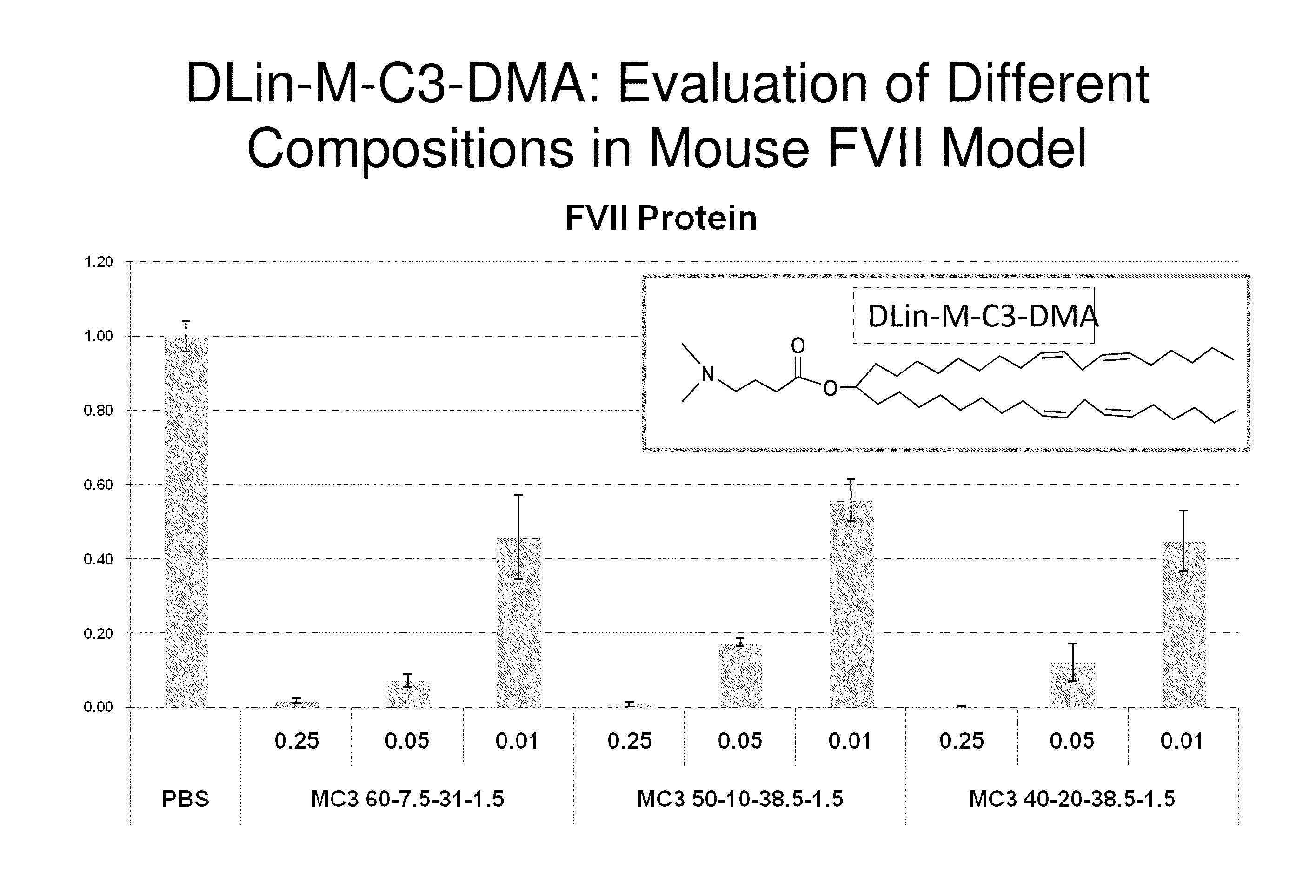
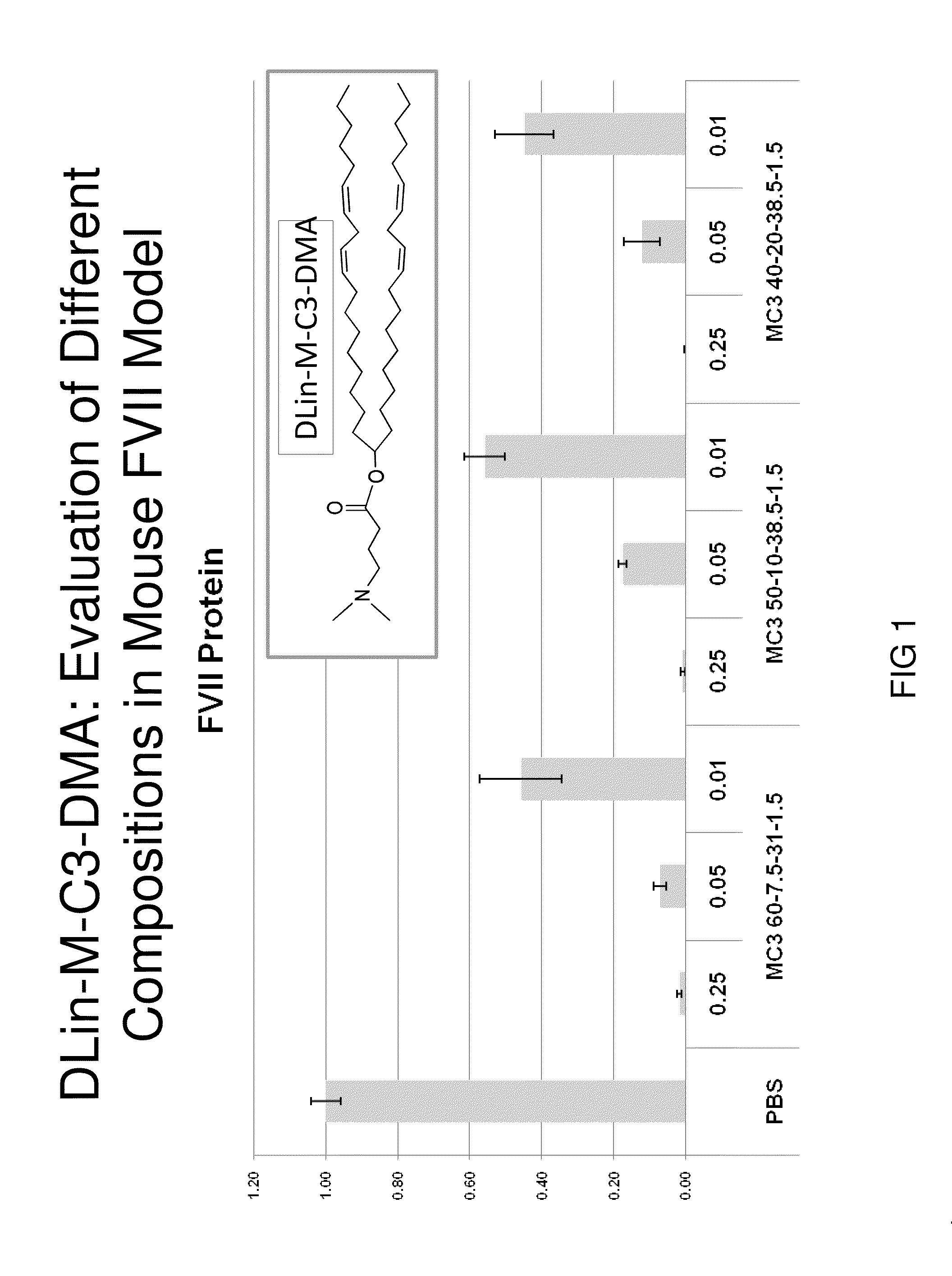
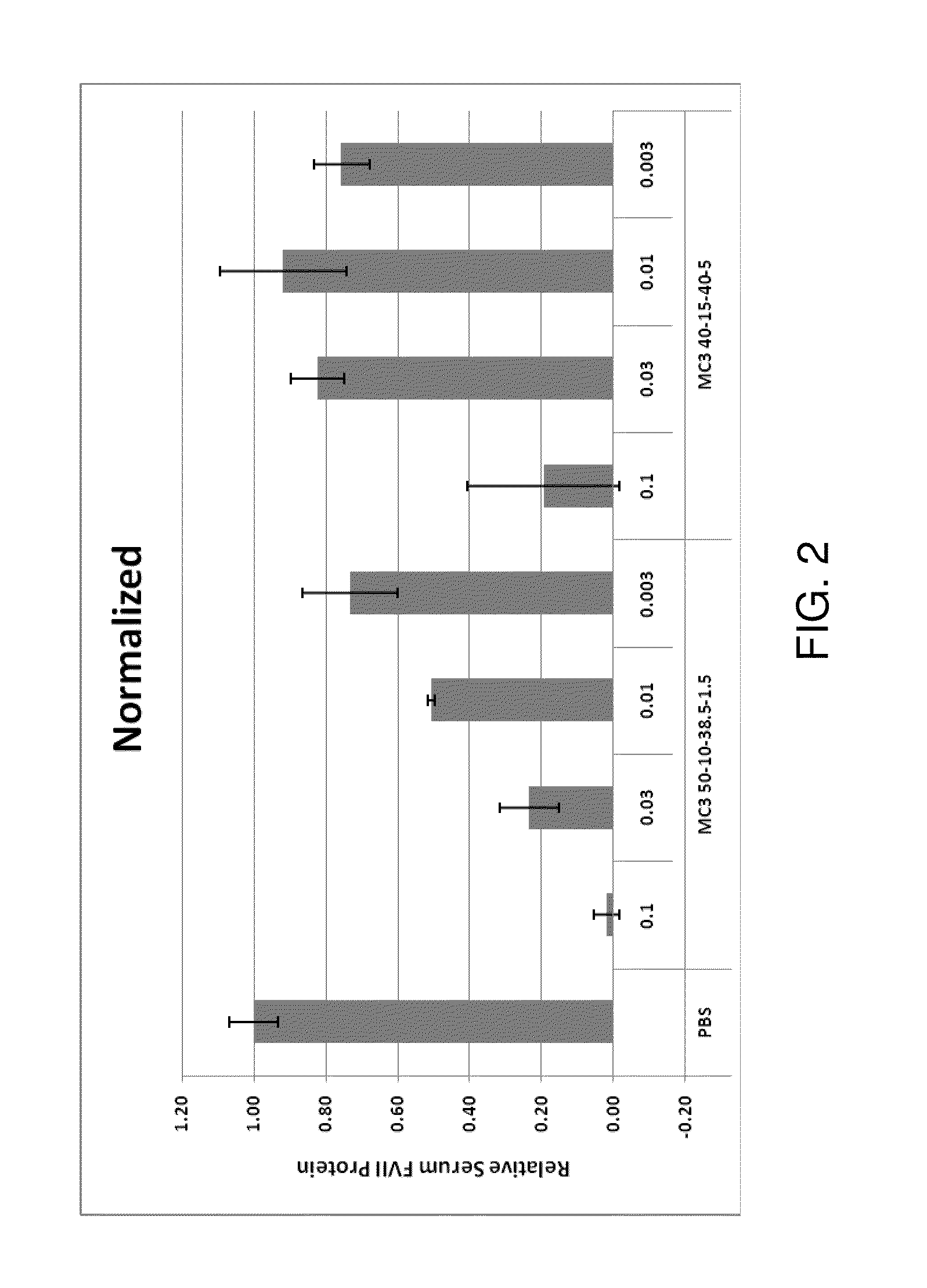
Lipid formulation
Owner:ARBUTUS BIOPHARMA CORPORAT ION
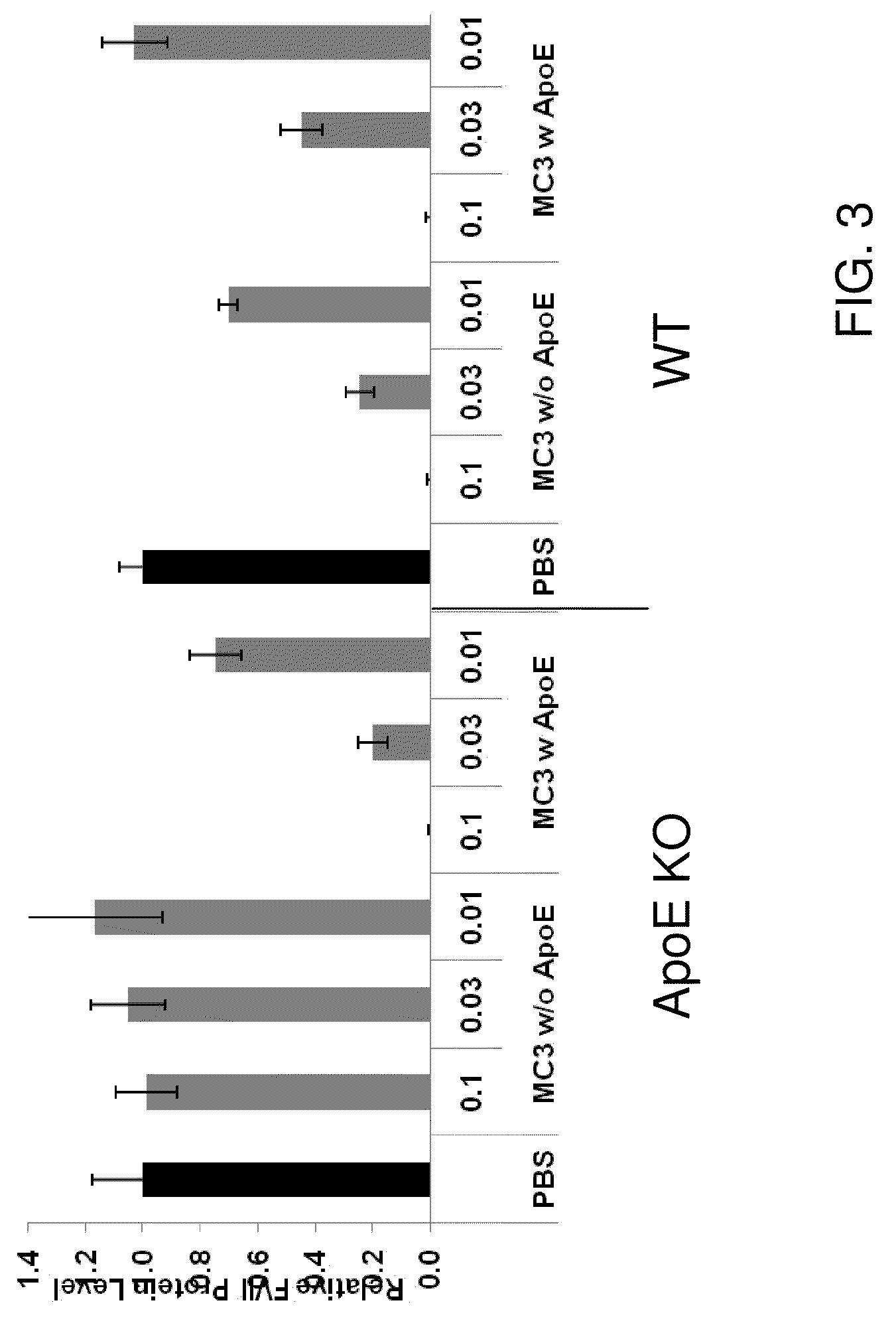
Lipid formulation
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Fused cyclooctyne compounds and their use in metal-free click reactions
InactiveUS8859629B2Easy to synthesizeAmenable to simple and straightforward modification of the various parts of the moleculeBiocideCarbamic acid derivatives preparationCombinatorial chemistry1,3-dipole
Owner:SYNAFFIX
Fused cyclooctyne compounds and their use in metal-free click reactions
InactiveUS20130137763A1Easy to synthesizeAmenable to simple and straightforward modification of the various parts of the moleculeBiocideCarbamic acid derivatives preparationCombinatorial chemistry1,3-dipole
The invention relates to fused cyclooctyne compounds, and to a method for their preparation. The invention also relates to a conjugate wherein a fused cyclooctyne compound according to the invention is conjugated to a label, and to the use of these conjugates in bioorthogonal labeling, imaging and / or modification, such as for example surface modification, of a target molecule. The invention further relates to a method for the modification of a target molecule, wherein a conjugate according to the invention is reacted with a compound comprising a 1,3-dipole or a 1,3-(hetero)diene.
Owner:SYNAFFIX
Production process and use for propargyl alcohol and its intermediate
InactiveUS6710213B2High yieldOrganic compound preparationPreparation by halogen eliminationAlcohol1-Propanol
Processes are provided for producing propargyl alcohol in an industrially advantageous manner. One of the processes comprises reaction of 1,2,3-trichloropropane with 3 equivalents or more of an alkali compound to produce propargyl alcohol. The other process comprises (1) a first step of reaction of 2,3-dichloro-1-propanol with an amine to produce chloroallyl alcohol, and (2) a second step of reaction of the chloroallyl alcohol obtained in the above step (1) with an alkali compound to produce propargyl alcohol.
Owner:RESONAC HOLDINGS CORPORATION
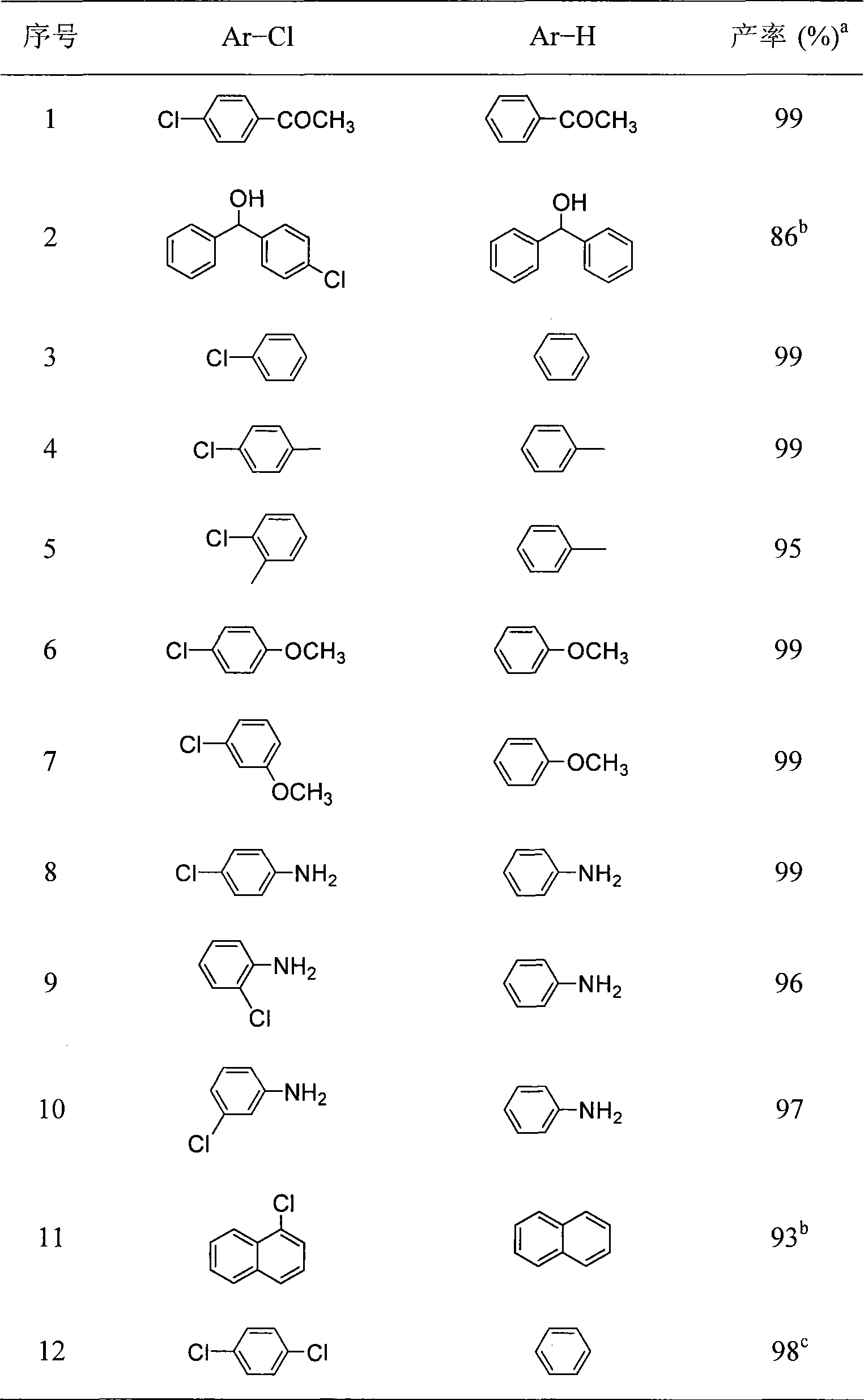
Room temperture nickel catalysis dechlorination method for chlorinated aromatic hydrocarbons
InactiveCN101475428AEasy to prepareHigh activityOrganic compound preparationPreparation by halogen eliminationAlcoholRoom temperature
The invention belongs to the technical field of fine chemical engineering and provides a method for dechlorinating chlorinated aromatic hydrocarbon through nickel catalysis at room temperature. The dechlorination reaction of the chlorinated aromatic hydrocarbon through nickel catalysis is characterized in that the reaction is carried out in a low-carbon alcoholic solvent in the presence of alkali under the condition of room temperature, and a reaction product is easy to separate and has high yield and good selectivity. The method has the advantages that the method uses low-carbon alcohol with low toxicity, low price and availability as a solvent and a divalent nickel complex with low price as a catalyst to catalyze the dechlorination reaction of the chlorinated aromatic hydrocarbon. The divalent nickel complex has a simple preparation method, high catalytic activity, good selectivity and stability in air.
Owner:DALIAN UNIV OF TECH
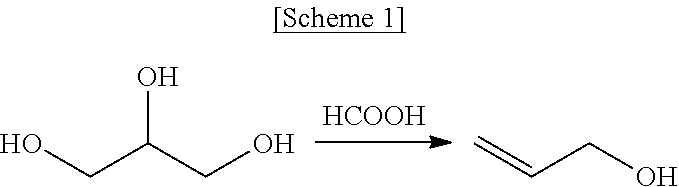
Method for producing allyl alcohol and allyl alcohol produced thereby
ActiveUS20160115109A1High yieldOxygen-containing compound preparationOrganic compound preparationRoom temperatureReaction temperature
Disclosed are a method of preparing allyl alcohol and allyl alcohol prepared thereby. The method of preparing allyl alcohol includes adding glycerol with formic acid in an amount of 0.8˜2 equivalents relative to 1 equivalent of glycerol, and increasing a reaction temperature to 220˜260° C. from room temperature at a heating rate of 2.0˜7.0° C. / min so that glycerol and formic acid are reacted.
Owner:LG CHEM LTD
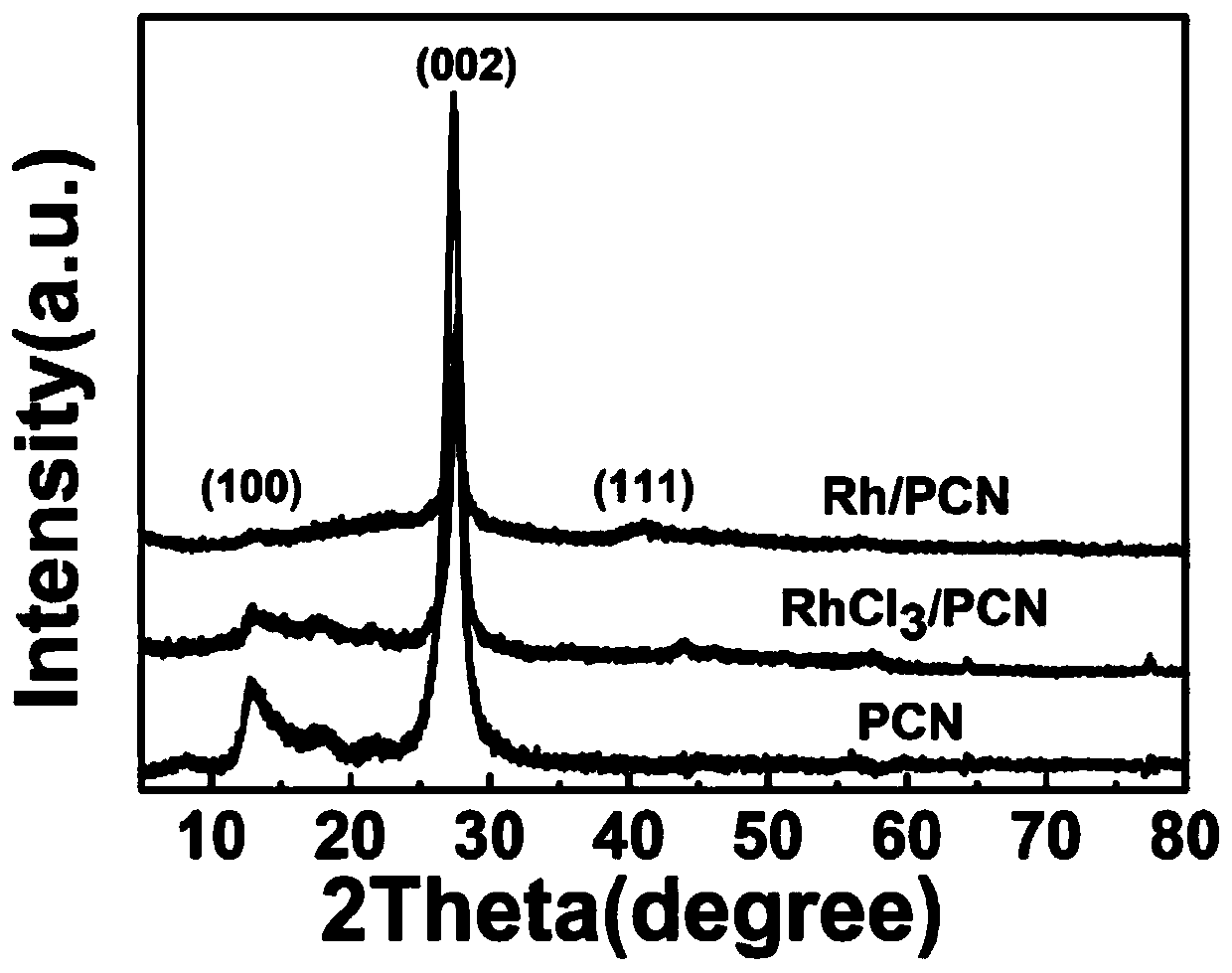
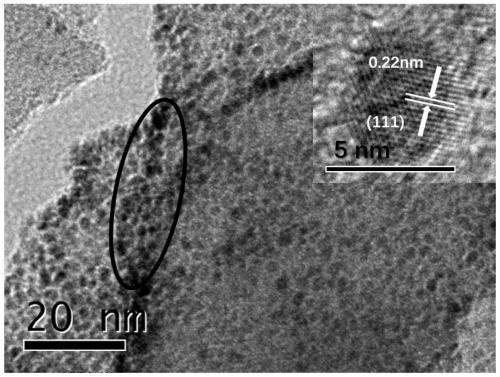
Preparation of rhodium loaded porous siphonate carbon nitride photocatalyst, and hydrodechlorination catalytic reaction of parachlorophenol of rhodium loaded porous siphonate carbon nitride photocatalyst
ActiveCN110385138AEasy to prepareHigh chemoselectivityPhysical/chemical process catalystsOrganic compound preparationCyclohexanonePhenol
The invention discloses preparation of a rhodium loaded porous siphonate carbon nitride photocatalyst, and a hydrodechlorination catalytic reaction of parachlorophenol of the rhodium loaded porous siphonate carbon nitride photocatalyst. The preparation method of the photocatalyst comprises the steps of taking melamine as a precursor to prepare carbon nitride by a thermal polymerization method, andthen in a mixed solution of ethyl alcohol and water (v / v 1 to 1), taking sodium borohydride as a reducing agent to prepare the rhodium loaded porous siphonate carbon nitride photocatalyst. The methodof hydrodechlorination of the parachlorophenol by photocatalyzing comprises the steps of putting a certain amount of catalyst and a certain concentration of chlorophenol aqueous solution to a glass reactor with a hydrogen balloon, reacting under illumination, and mainly generating phenol, cyclohexanone, cyclohexanol and other products. According to the preparation, the preparation method of the catalyst is simple, operation is easy, the catalyst can be used for photocatalysis chlorophenol to conduct the efficient hydrodechlorination, reaction conditions are mild, chemical selectivity of the cyclohexanol and the cyclohexanone is high, and the catalyst is easy to recycle.
Owner:CHONGQING TECH & BUSINESS UNIV
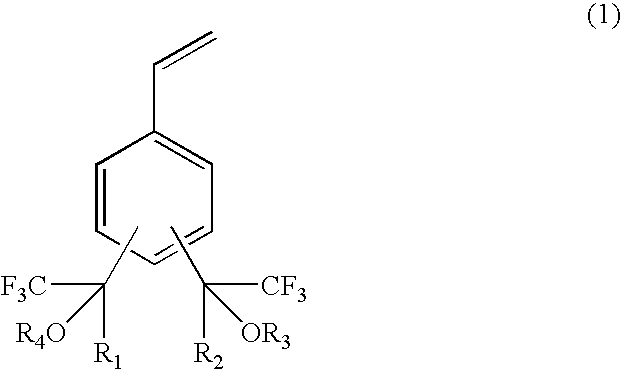
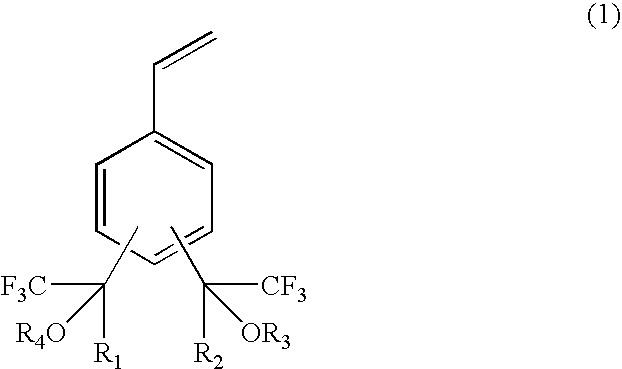
Fluorine-containing polymerizable monomers and polymers, anti-reflection film materials and resist compositions using same
InactiveUS7105618B2High transparencyLow refractive indexOrganic compound preparationPhotosensitive materialsResistHydrogen atom
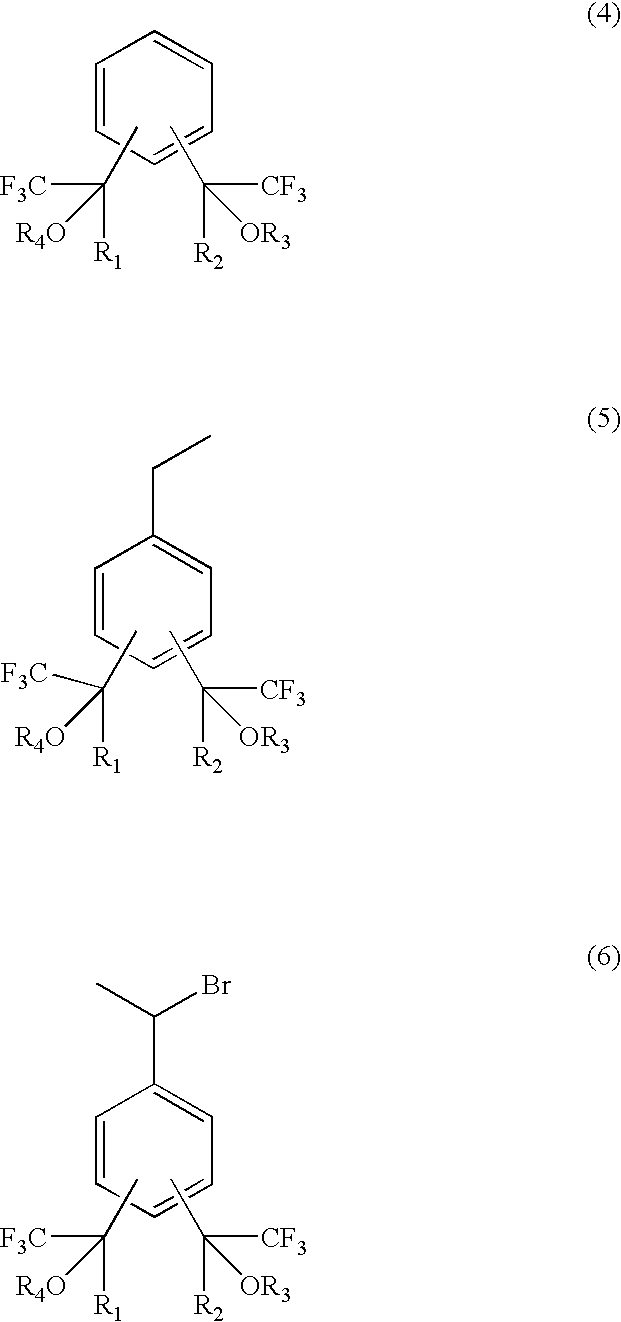
The present invention relates to a fluorine-containing polymerizable monomer having a structure of the general formula (1),where each of R1 and R2 is independently a methyl group or trifluoromethyl group, andeach of R3 and R4 is independently a hydrogen atom, an alkyl group, a fluorinated alkyl group, a ring structure having an aromatic ring, or an acid-labile protecting group, each of the alkyl group and the fluorinated alkyl group independently having a straight-chain, branched or ring form and having a carbon atom number of 1–25, each of R3 and R4 optionally and independently containing at least one of an oxygen atom and a carbonyl bond.
Owner:CENT GLASS CO LTD
Process for producing fluorine-containing, polymerizable styrene monomer and intermediates used in same
ActiveUS6943271B2Superior in industrially producingSilicon organic compoundsOrganic compound preparationHydrogenMetal catalyst
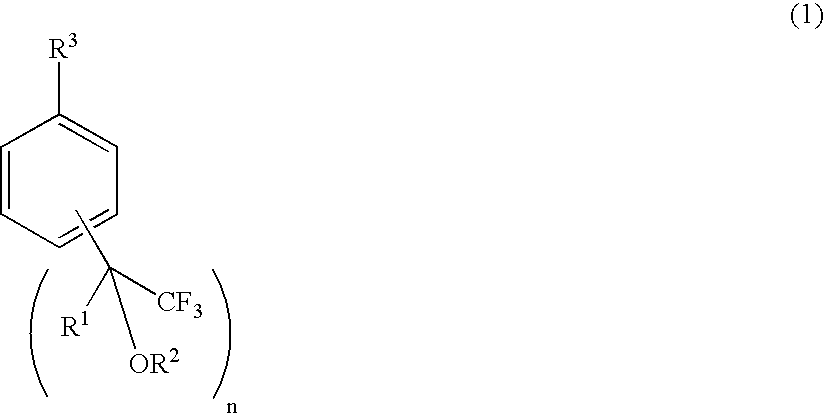
A fluorine-containing styrene monomer of the formula (2) is produced by a first, second or third process. The first process includes (a) reacting a compound of the formula (1) with a compound of the formula (3), in the presence of a metal catalyst; (b) reacting the product of the step (a) with a base; and (c) reacting the product of the step (b) with hydrogen, in the presence of a metal catalyst and a phosphine or amine, thereby producing the target styrene monomer. The second process includes reacting a compound of the formula (1) with a compound of the formula (12), in the presence of a metal catalyst, thereby producing the target styrene monomer. The third process includes reacting a compound of the formula (13) with a compound of the formula (14) or (15), in the presence of a base, thereby producing the target styrene monomer.
Owner:CENT GLASS CO LTD
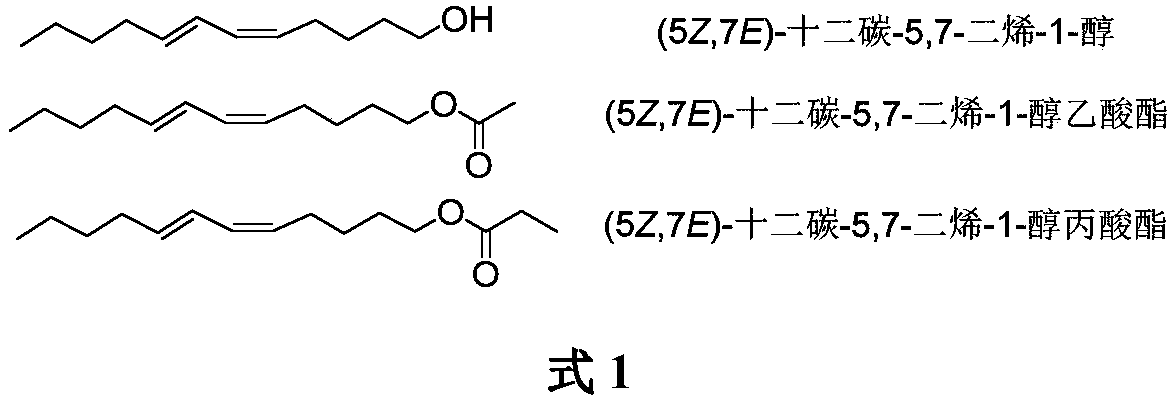
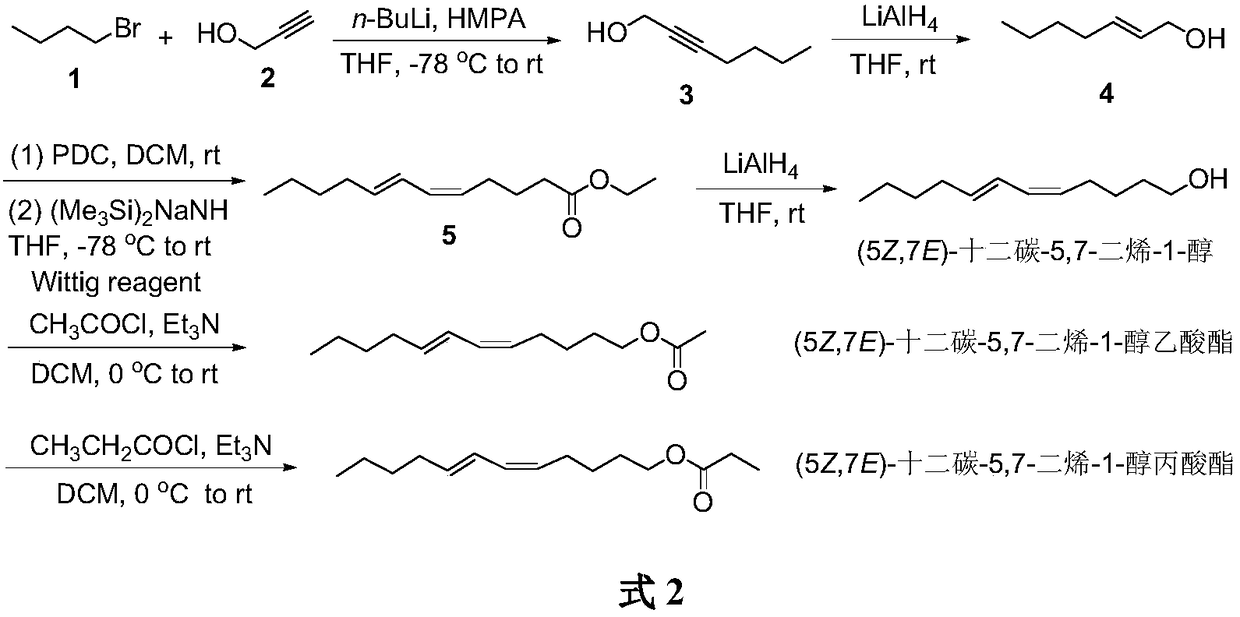
Synthesis of (5Z,7E)-dodecane-5,7-diene-1-alcohol as well as acetate and propionate thereof
ActiveCN109456182AOrganic compound preparationPreparation by hydrogenationTriphenyl phosphoniumEthyl Chloride
The invention belongs to the technical field of insect pheromone synthesis, and discloses a new method for synthesizing (5Z,7E)-dodecane-5,7-diene-1-alcohol as well as acetate and propionate thereof.The method takes propynol as an initial raw material to be subjected to coupling with 1-bromobutane to generate 2-heptyne-1-alcohol, triple bond is reduced to be E-type double bond through LiAlH4, 2-heptyne-1-alcohol is oxidized to be olefine aldehyde through PDC, olefine aldehyde reacts with a Wittig reagent (5-ethyoxyl-5-oxopentyl)triphenyl phosphonium bromide to produce (5Z,7E)-dodecane-5,7-dienoic acid ethyl ester which is reduced by LiAlH4 to obtain (5Z,7E)-dodecane-5,7-diene-1-alcohol, (5Z,7E)-dodecane-5,7-diene-1-alcohol reacts with acetyl chloride and propionyl chloride finally to obtain (5Z,7E)-dodecane-5,7-diene-1-alcohol acetate and (5Z,7E)-dodecane-5,7-diene-1-alcohol propionate. The method utilizes LiAlH4 to reduce the triple bond to be the E-type double bond, and utilizes theWittig reaction of the Wittig reagent with the tail end provided with ester group and aldehyde to directly build the Z-type double bond, the synthesis route is simple, convenient and efficient, and the reaction condition is moderate and environmentally friendly.
Owner:CHINA AGRI UNIV
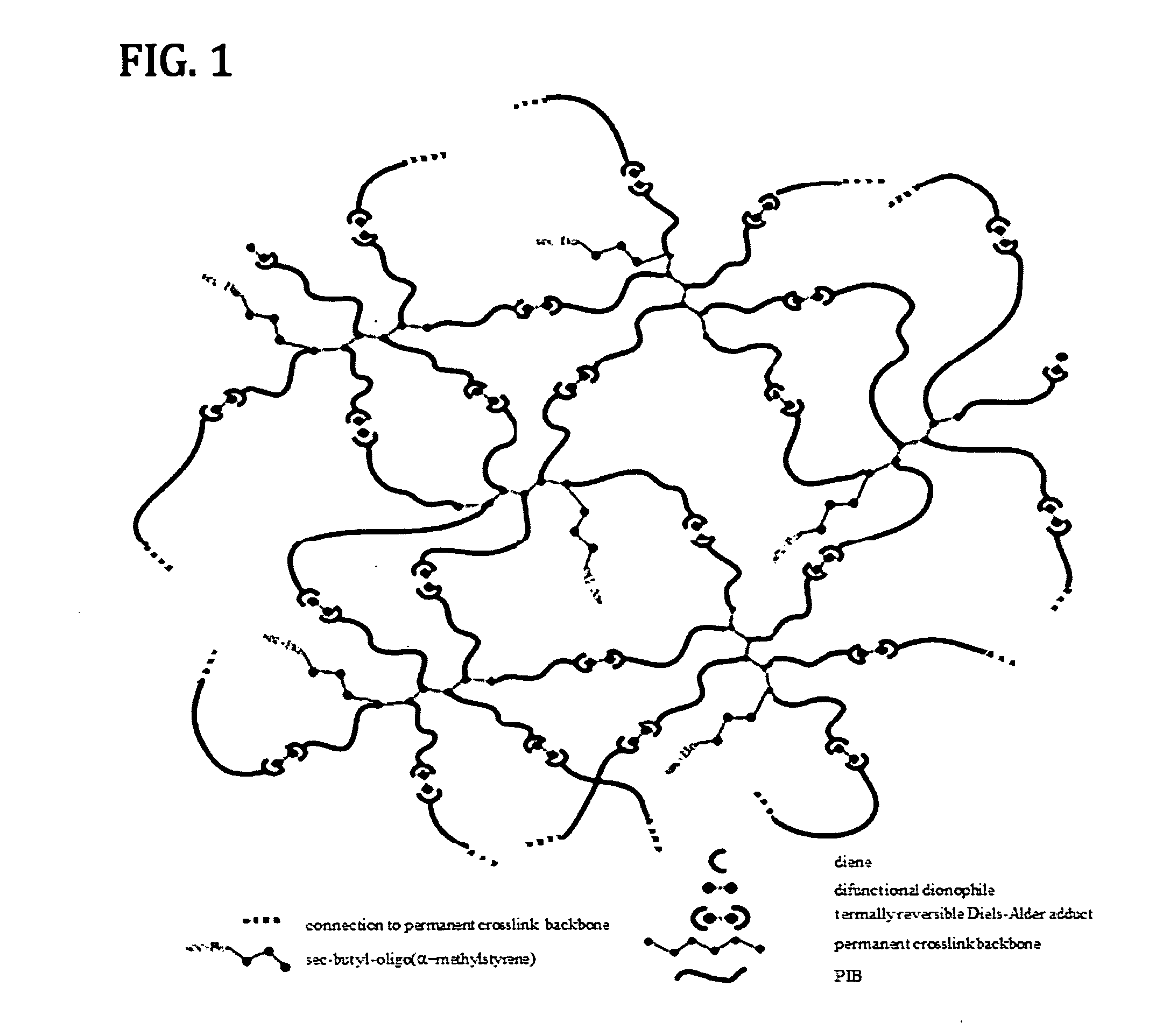
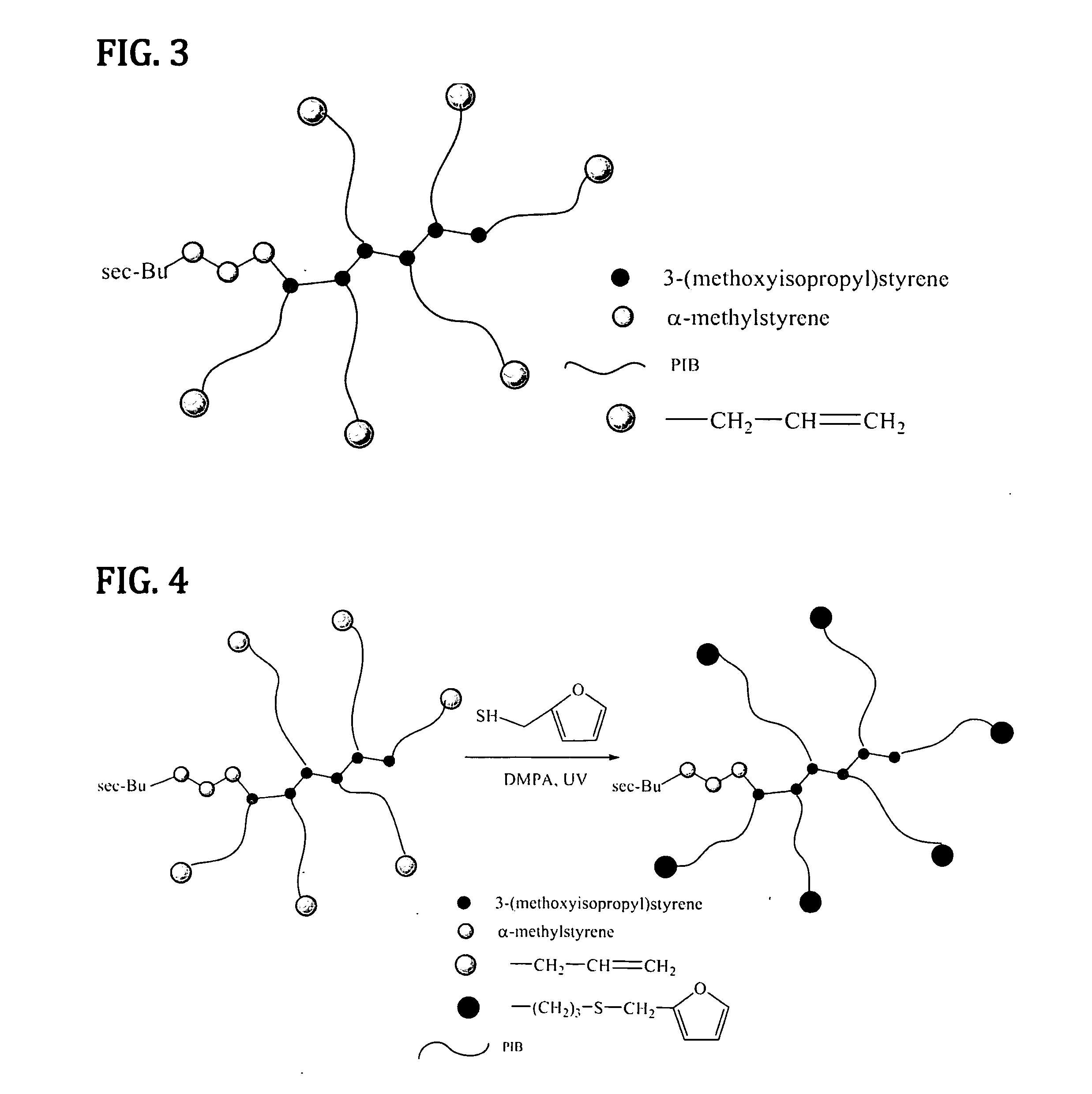
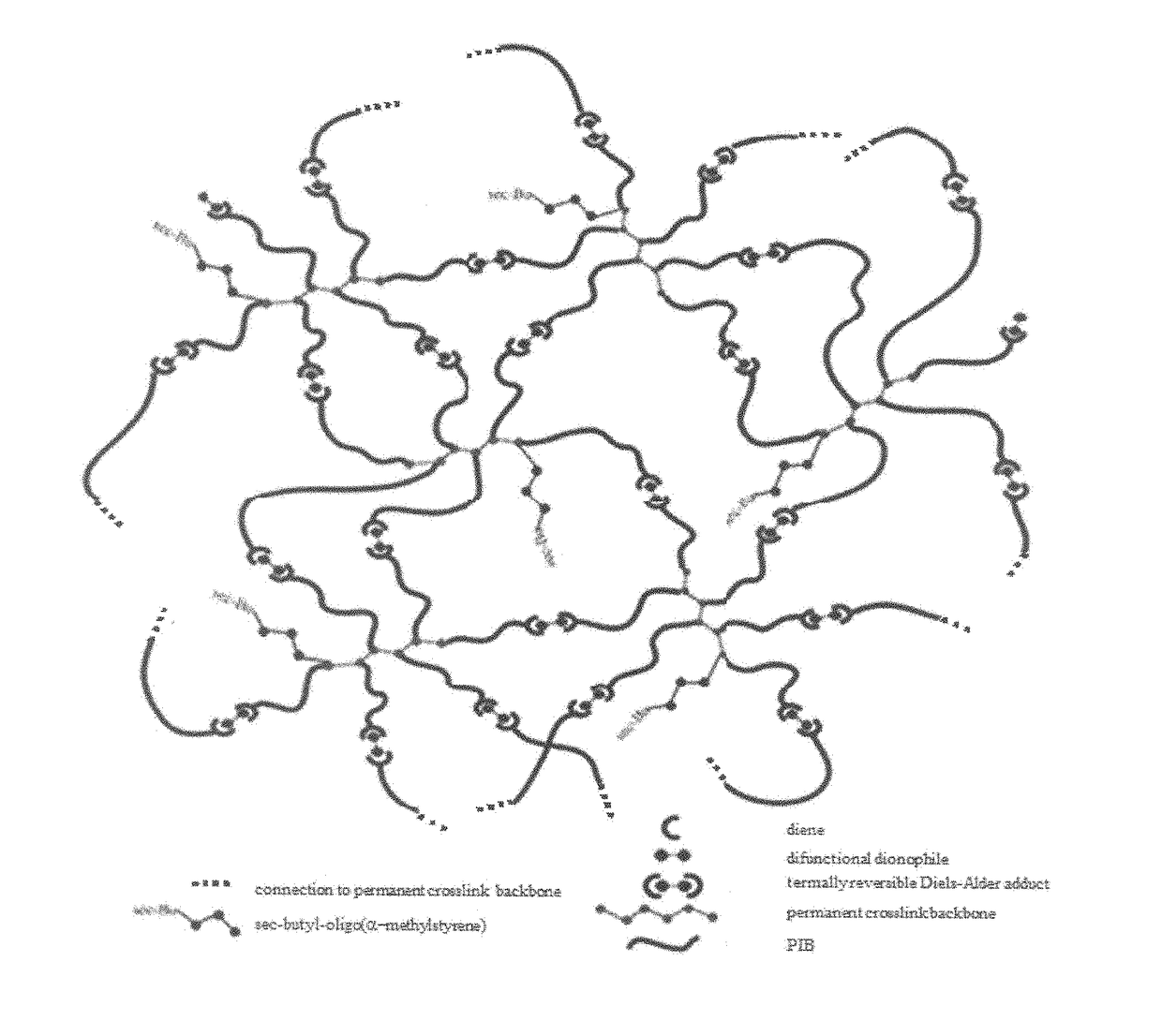
Novel Polyisobutylene-Based Thermoplastic Elastomers
InactiveUS20150210810A1Organic compound preparationPreparation by halogen eliminationChemical ligationThermoplastic elastomer
The present invention is directed to a new class of thermoplastic elastomers (TPEs) and processes for making them. In some embodiments of the present invention, the end groups of the multi-arm PIB copolymer is a conjugated diene, whereas the other component is a multi-functional dienophile. The components of the TPE of the present invention are chemically connected via the well-known Diels-Alder reaction which is thermally reversible (by the retro-Diels-Alder reaction) at moderately elevated temperatures. The reversibility of the Diels-Alder retro-Diels-Alder reactions allows the recovery of the original components of the TPE and thus its recyclability and also gives the TPE the ability to be reshaped or reformed.
Owner:THE UNIVERSITY OF AKRON
Preparation method of pentafluoropentanol
InactiveCN107501044AHigh reaction yieldEase of industrial productionPreparation by halogen introductionPreparation by halogen elimination1-PentanolReducing agent
The invention discloses a preparation method of pentafluoropentanol. The preparation method comprises the following steps: firstly, synthetic reaction: carrying out a reaction between propenol and pentafluoroethyliodide under the action of a free radical initiator to generate 4,4,5,5,5-pentafluoro-2-iodo-1-pentanol; secondly, hydrogenated dehalogenation reaction: carrying out the hydrogenated dehalogenation reaction under the action of the 4,4,5,5,5-pentafluoro-2-iodo-1-pentanol to generate a pentafluoropentanol product. The invention provides the preparation method of the pentafluoropentanol. The pentafluoroethyliodide and the propenol serving the raw material undergo free radical reaction to generate the 4,4,5,5,5-pentafluoro-2-iodo-1-pentanol, and the 4,4,5,5,5-pentafluoro-2-iodo-1-pentanol undergoes dehalogenation reaction under the action of a reducing agent to generate the pentafluoropentanol. The method is high in reaction yield and easy for industrial production; in addition, the purity of the product is higher than 99 percent.
Owner:天津长芦华信化工股份有限公司
Process and catalyst for converting alkanes
InactiveUS7812201B2Oxygen-containing compound preparationPreparation by halogen eliminationAlcoholEther
Owner:MILLER KLING HYDROCARBONS LLC
Method for preparing 4,4'-bis(hydroxymethyl)biphenyl
ActiveCN101607871ASimple processShort reaction timePreparation by halogen eliminationPreparation by hydrolysisSolventHydroxymethyl
The invention provides a method for preparing 4,4'-bis(hydroxymethyl)biphenyl, which comprises the following steps: mixing 4,4'-bis(chloromethyl)biphenyl, a solvent and alkaline liquor according to the mass ratio of 1:4-10:4-10; heating, refluxing and stirring the mixture under the normal pressure and at the temperature of 60 to 100 DEG C; continuously reacting the mixture for at least 4 hours, adding water into the mixture, and filtrating the mixture to obtain a crude product of the 4,4'-bis(hydroxymethyl)biphenyl; and recrystallizing the crude product by an alcoholic solvent to obtain a fine product of the 4,4'-bis(hydroxymethyl)biphenyl. The method has simple operation and short reaction time and is suitable for industrialized production; and the solvent for reaction and the solvent for crystallization can be recycled.
Owner:BAOWU CHARCOAL MATERIAL TECH CO LTD
Method for synthesizing propargyl alcohol
ActiveCN107032956AReduce the difficulty of dehydrationPreparation by halogen eliminationPreparation by hydrolysisAlcoholSolvent
The invention discloses a method for synthesizing propargyl alcohol. The method includes hydrolyzing 1, 3-dichloropropene in saturated alkali metal carbonate solution to obtain 3-chloropropene alcohol; removing hydrogen chloride from the 3-chloropropene alcohol under the condition of the presence of a polar solvent dimethyl sulfoxide and hydroxide of alkali metal so as to obtain the propargyl alcohol. The method has the advantages that the 1, 3-dichloropropene is used as a raw material, the raw material can come from wide sources, is inexpensive and is easily available, the propargyl alcohol is easy to dehydrate, energy can be saved, and environments can be protected; the propargyl alcohol obtained by the aid of the method is high in yield and purity.
Owner:JINGCHU UNIV OF TECH
Preparation method of 1, 1-cyclopropane dimethanol
InactiveCN102757311ASimple processImprove securityOrganic compound preparationPreparation by halogen eliminationAlcoholCyclopropane
The invention relates to a preparation method of 1, 1-cyclopropane dimethanol. The method comprises the steps of conducting ring-closure reaction on organic dihalide and a reducing agent in an alcohol solvent under a certain condition to obtain a solution of 1, 1-cyclopropane dimethanol and halide salt, introducing ammonia under certain conditions so as to conduct solid-liquid separation, filter and rectify to obtain 1, 1-cyclopropane dimethanol. Due to the adoption of the preparation method, the process of 1, 1-cyclopropane dimethanol is simple and concise, the safety is high, and meanwhile, the yield reaches to more than 90%, the purity of 1, 1-cyclopropane dimethanol is more than 98%, and the quality of 1, 1-cyclopropane dimethanol is improved effectively.
Owner:ZHEJIANG HUTU PHARMCHEM
Processes for the preparation of cinacalcet
InactiveUS20120309842A1Non-expensiveReadily availableBiocideOrganic active ingredientsCombinatorial chemistry
The present invention provides processes and intermediates for preparing cinacalcet base and pharmaceutically acceptable salts thereof.
Owner:RANBAXY LAB LTD
Synthetic method for 2-butyne-1-ol
InactiveCN104045514ASave raw materialsWide variety of sourcesOrganic compound preparationPreparation by halogen eliminationPulp and paper industryHydrolysis
The invention discloses a synthetic method for 2-butyne-1-ol. Innovation points of the invention are as follows: a waste liquid containing 1,3-dichloro-2-butylene and produced in rubber production is used as a starting raw material and undergoes hydrolysis so as to obtain 3-chloro-2-butenyl-1-ol, then elimination is carried out so as to obtain 2-butyne-1-ol, and finally high-purity 2-butyne-1-ol is prepared. According to the synthetic method for 2-butyne-1-ol, the raw material is the waste liquid produced in rubber production and is cheap and widely available, so utilization of the waste, greenness and environmental protection are realized. The synthetic method is simple and is safe and easy to operate; and consumed labor force and resources by the method are substantially reduced. Product yield reaches more than 88%, and product purity reaches 96 to 98%.
Owner:姜树林
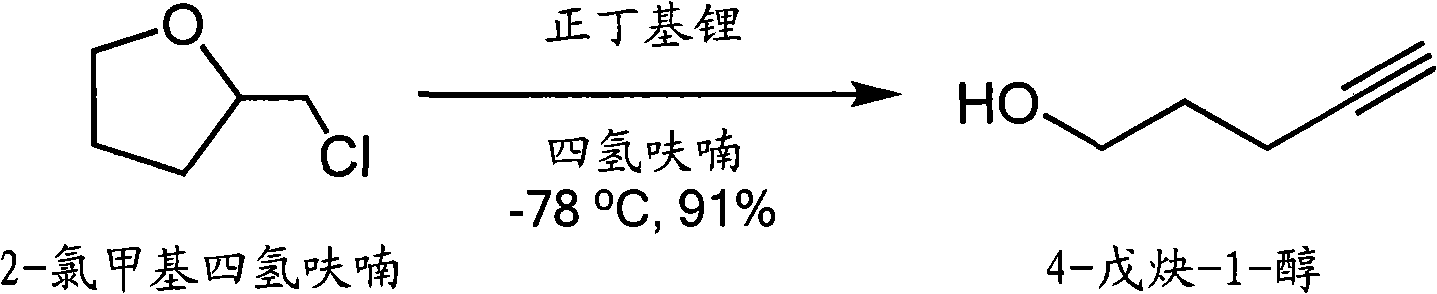
Preparation method of 4-pentyne-1-alcohol
The invention relates to a new method for synthesizing 4-pentyne-1-alcohol. In the method, 2-chloromethyl tetrahydrofuran is used as a raw material, in the presence of a lower temperature, tetrahydrofuran is used as a solvent, and n-butyllithium is used as a strong base for eliminating to obtain the 4-pentyne-1-alcohol, wherein the productivity can reach 91 percent. In the invention, the low-price raw material and a simple and convenient condition are used, and the method has higher productivity compared with the traditional method.
Owner:周雄飞
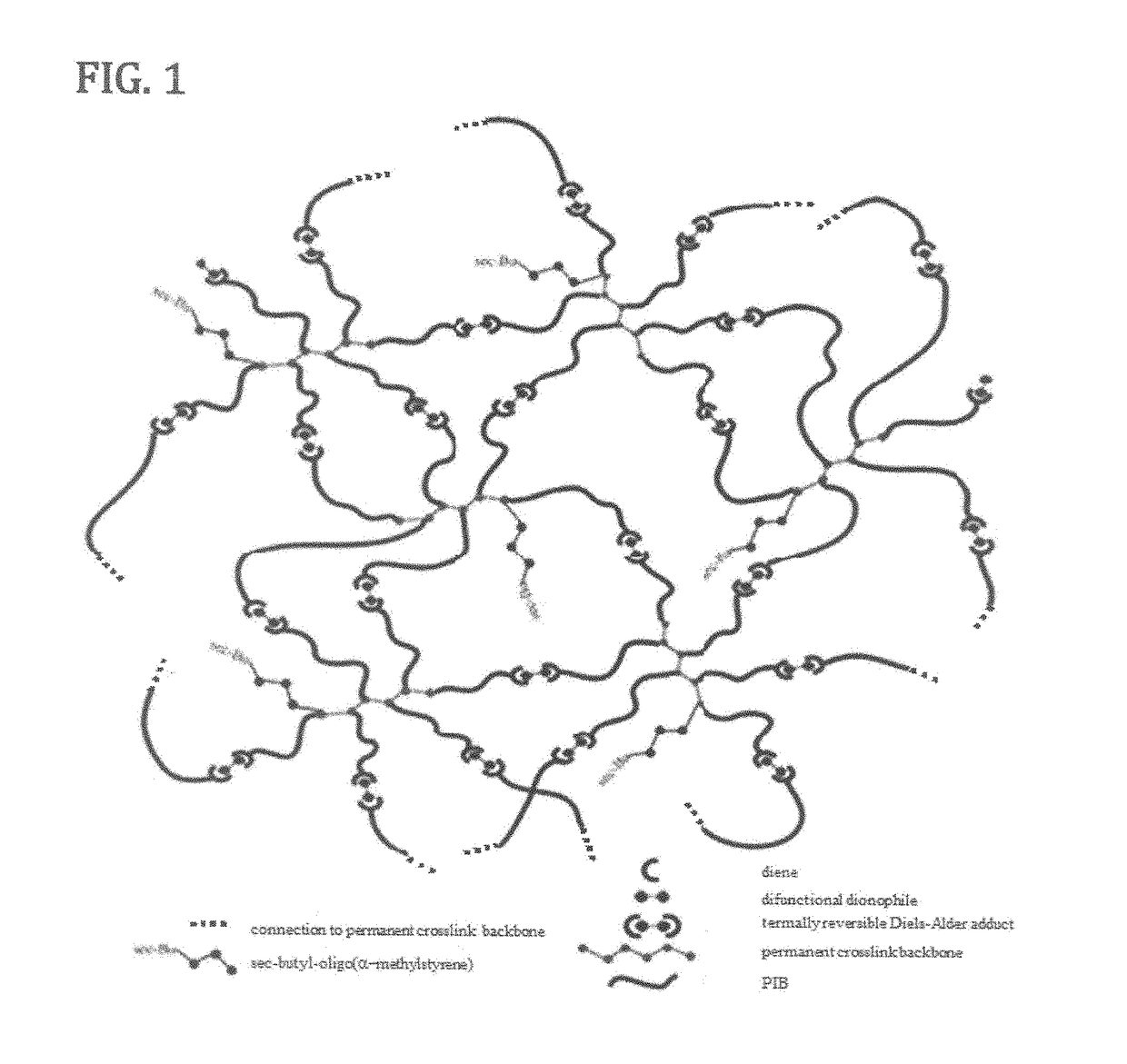
Novel polyisobutylene-based thermoplastic elastomers
ActiveUS20170240493A1Preparation by halogen eliminationPreparation of metal alcoholatesChemical ligationEnd-group
The present invention is directed to a new class of thermoplastic elastomers (TPEs) and processes for making them. In some embodiments of the present invention, the end groups of the multi-arm PIB copolymer is a conjugated diene, whereas the other component is a multi-functional dienophile. The components of the TPE of the present invention are chemically connected via the well-known Diels-Alder reaction which is thermally reversible (by the retro-Diels-Alder reaction) at moderately elevated temperatures. The reversibility of the Diels-Alder retro-Diels-Alder reactions allows the recovery of the original components of the TPE and thus its recyclability and also gives the TPE the ability to be reshaped or reformed.
Owner:THE UNIVERSITY OF AKRON
Production process and use for propargyl alcohol and its intermediate
InactiveUS20020010377A1Improve moisture resistanceLow curing temperatureOrganic compound preparationPreparation by halogen eliminationAlcohol1-Propanol
Processes are provided for producing propargyl alcohol in an industrially advantageous manner. One of the processes comprises reaction of 1,2,3-trichloropropane with 3 equivalents or more of an alkali compound to produce propargyl alcohol. The other process comprises (1) a first step of reaction of 2,3-dichloro-1-propanol with an amine to produce chloroallyl alcohol, and (2) a second step of reaction of the chloroallyl alcohol obtained in the above step (1) with an alkali compound to produce propargyl alcohol.
Owner:SHOWA DENKO KK
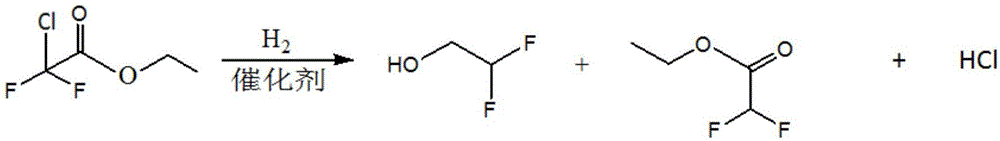
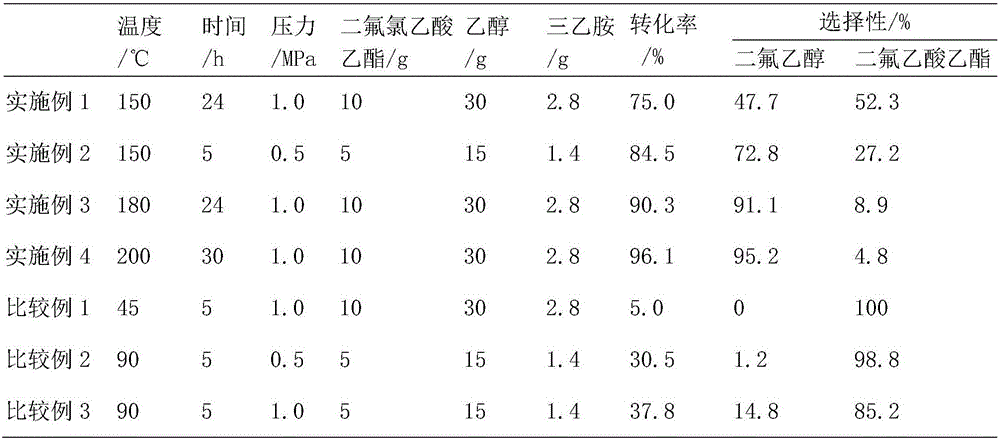
Method for preparing difluoroethanol through catalytic hydrogenation
InactiveCN106316795AImprove conversion rateLow hydrogen ester ratioOrganic compound preparationPreparation by halogen eliminationHydrogenation reactionAsymmetric hydrogenation
The invention provides a method for preparing difluoroethanol through catalytic hydrogenation. The method has the beneficial effects that hydrogenation reduction reaction is carried out by using ethyl chlorodifluoroacetate as the raw material, a Pd / C as a catalyst, triethylanmine as an acid-binding agent and ethanol as a solvent; the atom F has higher electronegativity and HF can not be generated in the ethyl chlorodifluoroacetate hydrogenation process, but the C-Cl bond is easy to hydrogenate and HCl is easy to generate, thus neutralizing generated HCl by adding the acid-binding agent triethylanmine, propelling reaction to be carried out positively and increasing the conversion rate of ethyl chlorodifluoroacetate; on the other side, used in ester hydrogenation reaction, the Pd / C catalyst has good characteristics of low hydrogen-ester ratio, high raw material conversion rate, high alcohol selectivity, stable catalyst, and the like; and therefore, the method has the effect of solving the problems that the reaction process in the traditional ester hydrogenation process is complex, is high in investment and is not suitable for small-scale production in the fine chemical industry field.
Owner:ZHEJIANG UNIVERSITY OF SCIENCE AND TECHNOLOGY
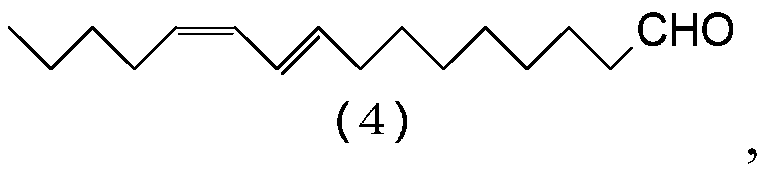
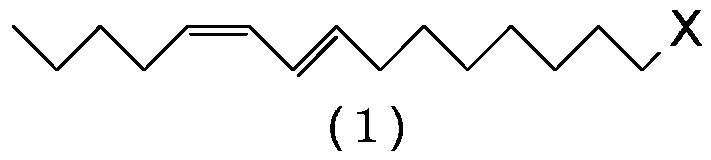
Process for preparing (9e,11z)-9, 11-hexadecadienal
PendingCN110386865AReduce environmental burdenHigh yieldOrganic compound preparationOrganic chemistry methodsNucleophilic substitutionAlkyl substitution
Owner:SHIN ETSU CHEM IND CO LTD
Synthetic navel orangeworm pheromone composition and methods relating to production of same
ActiveUS7737306B2Improve stabilityOrganic compound preparationPreparation by halogen eliminationNavelStereochemistry
One or more embodiments of the invention are directed to the synthetic methods for making lepidopteran pheromones including navel orangeworm pheromones. The synthetic methods involve novel, efficient, and environmentally benign steps and procedures.
Owner:SUTERRA
Processes for the preparation of cinacalcet
InactiveUS8759586B2Non-expensiveReadily availableBiocideOrganic active ingredientsCombinatorial chemistryCinacalcet
Owner:RANBAXY LAB LTD
Preparation method of 2,3,5,6-tetrafluorohydrazine-1,4-benzene dimethanol
ActiveCN106565421ARaw materials are cheap and easy to getShort synthetic routePreparation by halogen eliminationHalogenated hydrocarbon preparationChemical synthesisDistillation
The invention discloses a preparation method of 2,3,5,6-tetrafluorohydrazine-1,4-benzene dimethanol, and belongs to the technical field of fine chemical synthesis. The preparation method is characterized by comprising the following reaction steps that (1) chlorine is led into paraxylene under catalyzing of a catalyst and irradiation of visible light to achieve chlorination, then nitrogen is led in to expel residual hydrogen chloride gas, and 1,4-dual(trichloromethyl)-2,3,5,6-tetrachlorobenzene is obtained; (2) the 1,4-dual(trichloromethyl)-2,3,5,6-tetrachlorobenzene and potassium fluoride are subject to fluorization and alcoholysis in an alcohol solvent, the solvent is recycled through reduced pressure distillation, washing is carried out the neutral state is obtained, drying is carried out, and 1,4-dual(silane oxygen methyl chloride)-2,3,5,6-tetrachlorobenzene is obtained; and (3) hydrogen is led into the 1,4-dual(silane oxygen methyl chloride)-2,3,5,6-tetrachlorobenzene in a solvent containing a metal catalyst and an acid-binding agent to be subject to catalytic hydrogenation reduction, an upper-layer organic phase is subject to cooling, suction filtration and drying to obtain the2,3,5,6-tetrafluorohydrazine-1,4-benzene dimethanol. The preparation method has the beneficial effects that the raw materials are low in price and easy to obtain, the synthetic route is short, operation is easy and convenient, and the product cost is low.
Owner:岳阳中科华昂精细化工科技有限公司 +1
Side fluorine-containing diphenyl acetylene diluent for liquid crystals with high birefringence and synthesis method of side fluorine-containing diphenyl acetylene diluent
ActiveCN107628932AImprove low temperature performanceLow rotational viscosityPreparation by halogen eliminationHalogenated hydrocarbon preparationCrystallographySynthesis methods
The invention discloses a side fluorine-containing diphenyl acetylene diluent for liquid crystals with high birefringence and a synthesis method of the side fluorine-containing diphenyl acetylene diluent. The structural formula of the diluent is shown in the description, wherein X represents O or CH2 and R represents C1-C6 alkyl; the diluent is synthesized by industrially mature Sonogashira coupling and Witting reaction. The method is simple and efficient and is suitable for industrialized production. The diluent structurally contains a side fluorine substituent and a terminal alkene substituent, the low-temperature performance of the liquid crystals with high birefringence can be effectively improved, the birefringence of the diluent is higher than that of a commercial 3HHV diluent by 13-15 times, and the high birefringence of a liquid crystal material can be guaranteed while rotational viscosity of the liquid crystals with high birefringence is reduced.
Owner:SHAANXI NORMAL UNIV
Synthesis of pheromones and related materials via olefin metathesis
ActiveUS20200039900A1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPolymer sciencePtru catalyst
Methods for preparation of olefins, including 8- and 11-unsaturated monoenes and polyenes, via transition metathesis-based synthetic routes are described. Metathesis reactions in the methods are catalyzed by transition metal catalysts including tungsten-, molybdenum-, and ruthenium-based catalysts. The olefins include insect pheromones useful in a number of agricultural applications.
Owner:PROVIVI
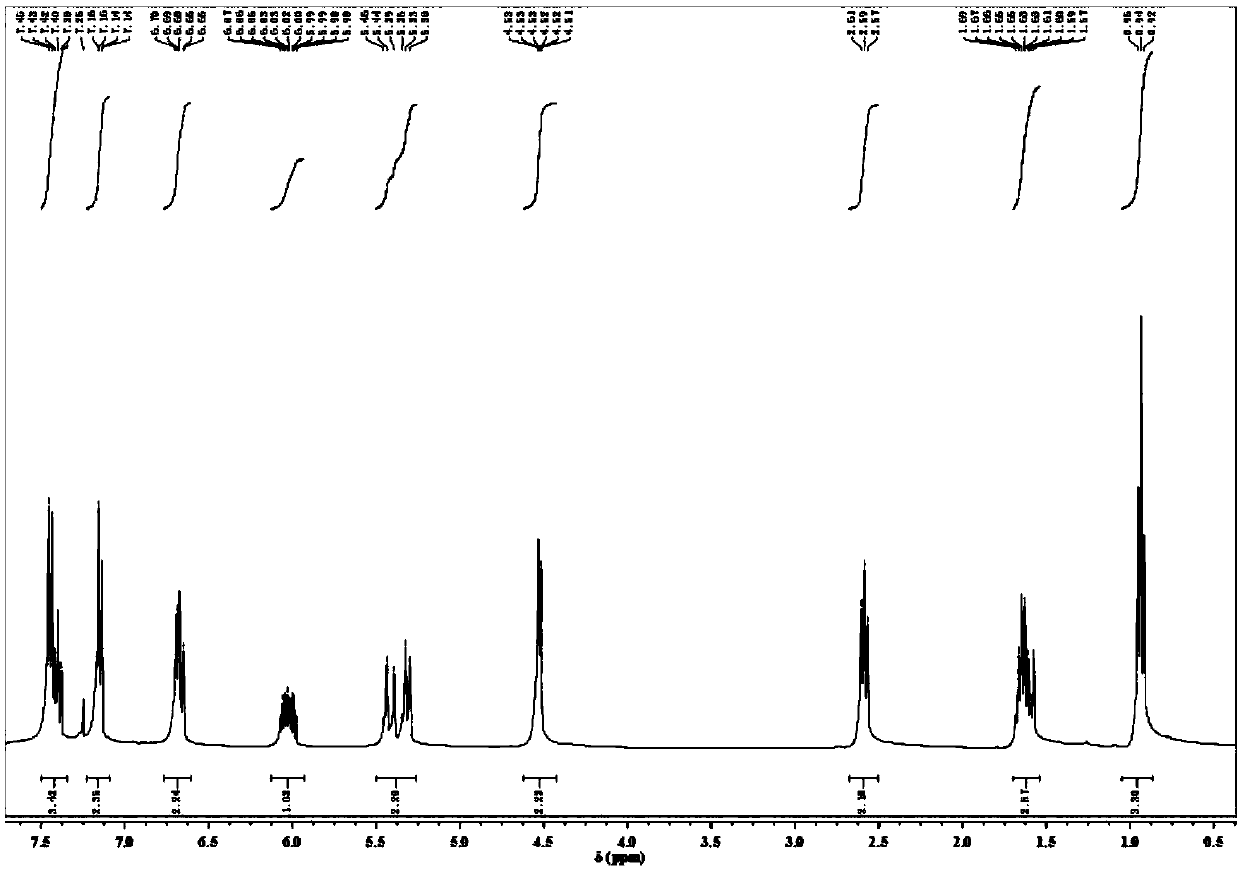
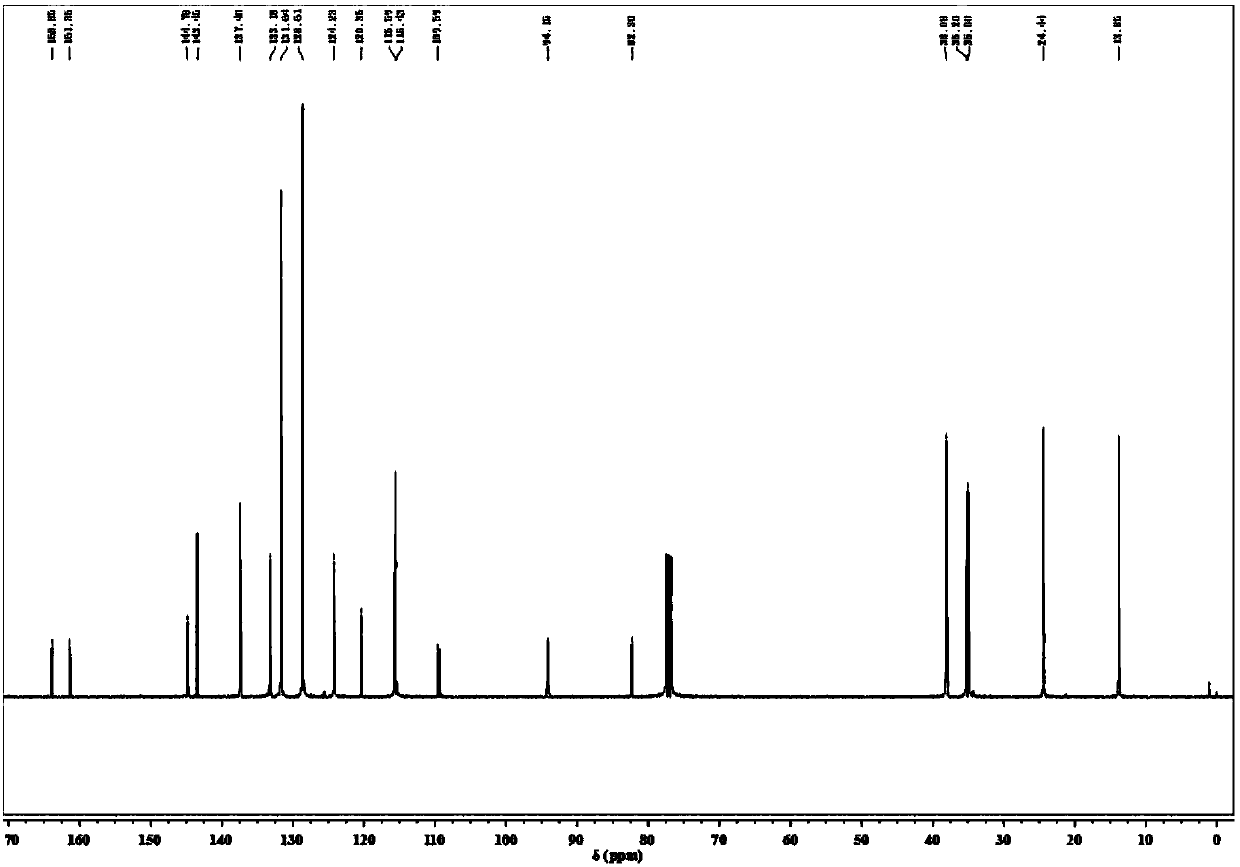
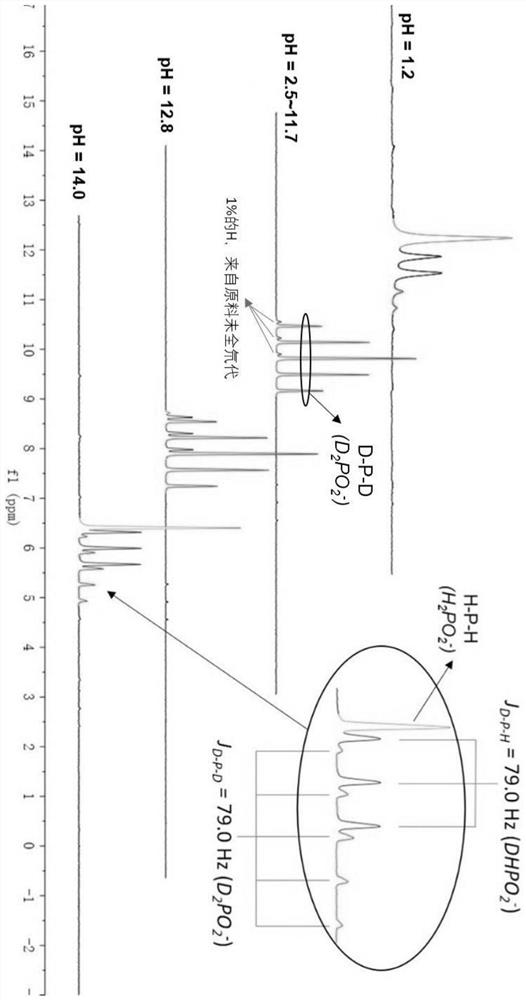
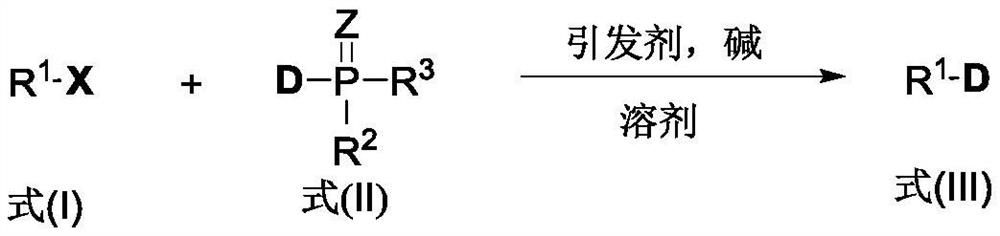
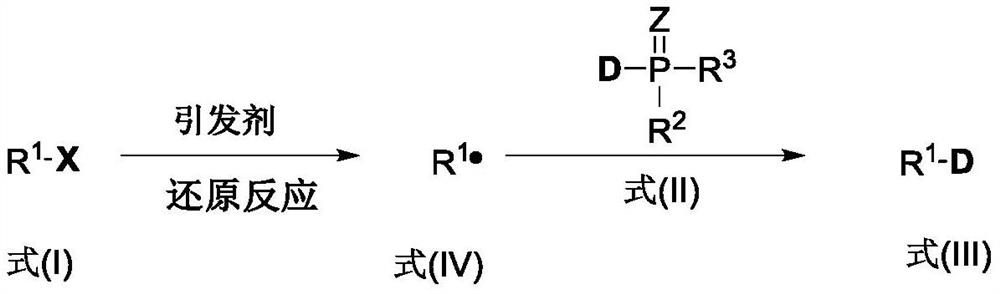
Method for synthesizing deuterated compound in aqueous phase solvent
ActiveCN112778379AEasy to operateGood substrate applicabilityEsterified saccharide compoundsIsotope introduction to sugar derivativesChemical synthesisChemical reaction
The invention belongs to the technical field of isotope labeling chemical synthesis, and discloses a method for synthesizing a deuterated compound in an aqueous phase solvent. The method is based on the following chemical reaction equation: a halide, diazonium salt or Xanthate shown in a formula (I), a material containing a phosphorus-deuterium bond shown in a formula (II), an initiator, alkali and a solvent are mixed and react to obtain a reduced deuterated product shown in a formula (III), wherein the solvent is water (1H2O) or a mixed solvent consisting of water and an organic solvent. By researching and improving the types of raw materials for constructing a reaction system and adopting the material containing the phosphorus-deuterium bond shown in the formula (II) as a deuterium source, compared with the prior art, the method can effectively solve the problems that the reaction conditions are strict, the adopted deuterium source is seriously excessive, strict anhydrous (1H2O) is needed and / or the reaction needs to be carried out in a deuterated solvent in the preparation process of the deuterated compound.
Owner:HUAZHONG UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com