Room temperture nickel catalysis dechlorination method for chlorinated aromatic hydrocarbons
A chlorinated aromatic hydrocarbon and nickel-catalyzed technology, which is applied in chemical instruments and methods, organic chemistry, carbon-based compound preparation, etc., can solve the problems of high cost, high reaction temperature, use of strong alkali, etc., and achieves easy separation and simple preparation method , highly active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Step 1 Connect the 20mL reaction bottle equipped with a stirring magnet with ultra-pure nitrogen, and repeat the evacuation and nitrogen filling three times, and then perform subsequent operations according to the method for handling sensitive substances in the air.
[0017] Step 2 Add 3mL of deoxygenated and dehydrated isopropanol to the reaction flask, then, chlorinated aromatic hydrocarbon (1.0mmol), potassium phosphate monohydrate (2.0mmol) and catalyst trans-Ni(PPh 3 ) 2 (1-Nap)Cl (0.03mmol), PCy 3 ·HBF 4 (0.06mmol) was added to the bottle in turn, stirred and reacted at room temperature for a certain period of time, and the reaction progress was monitored by thin-layer plates or gas chromatography.
[0018] Step 3 After the reaction, the catalyst was removed through a short silica gel column, and the product was purified by column chromatography, using a mixture of n-hexane and ethyl acetate as the developing solvent.
Embodiment 2
[0020] Step 1 is the same as in Example 1.
[0021] Step 2 Add 3mL isopropanol solvent to the reaction flask, then, chlorinated aromatic hydrocarbon (1.0mmol), potassium phosphate trihydrate (2.0mmol) and catalyst trans-Ni(PPh 3 ) 2 (1-Nap)Cl (0.03mmol), PCy 3 ·HBF 4 (0.06mmol) was added to the bottle in turn, stirred and reacted at room temperature for a certain period of time, and the reaction progress was monitored by thin-layer plates or gas chromatography.
[0022] Step 3 is the same as Embodiment 1.
Embodiment 3
[0024] Step 1 is the same as in Example 1.
[0025] Step 2 Add 3mL of deoxygenated isopropanol to the reaction flask, then, chlorinated aromatic hydrocarbon (1.0mmol), potassium phosphate monohydrate or potassium phosphate trihydrate (2.0mmol) and catalyst trans-Ni(PCy 3 ) 2 (1-Nap)Cl (0.03mmol) was sequentially added into the bottle, stirred and reacted at room temperature for a certain period of time, and the reaction progress was monitored by thin-layer plate or gas chromatography.
[0026] Step 3 is the same as Embodiment 1.
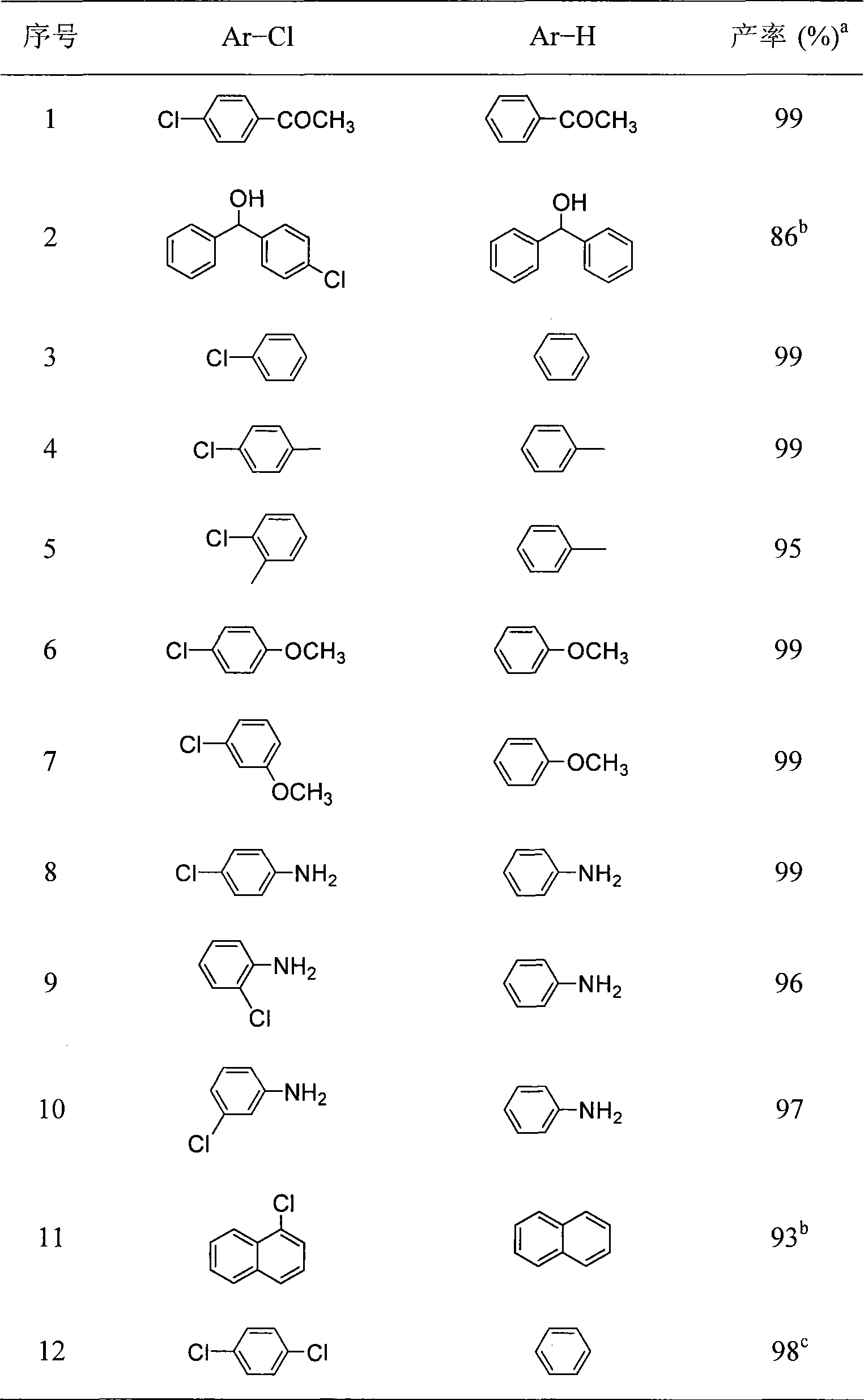
[0027] The reaction results are shown in Table 1.
[0028] Table 1 Results of dechlorination of chlorinated aromatics catalyzed by divalent nickel complexes
[0029]
[0030] Note: The data in the table were reacted at room temperature, a is the gas phase yield, b is the isolated yield, and c used 3 equivalents of alkali.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com