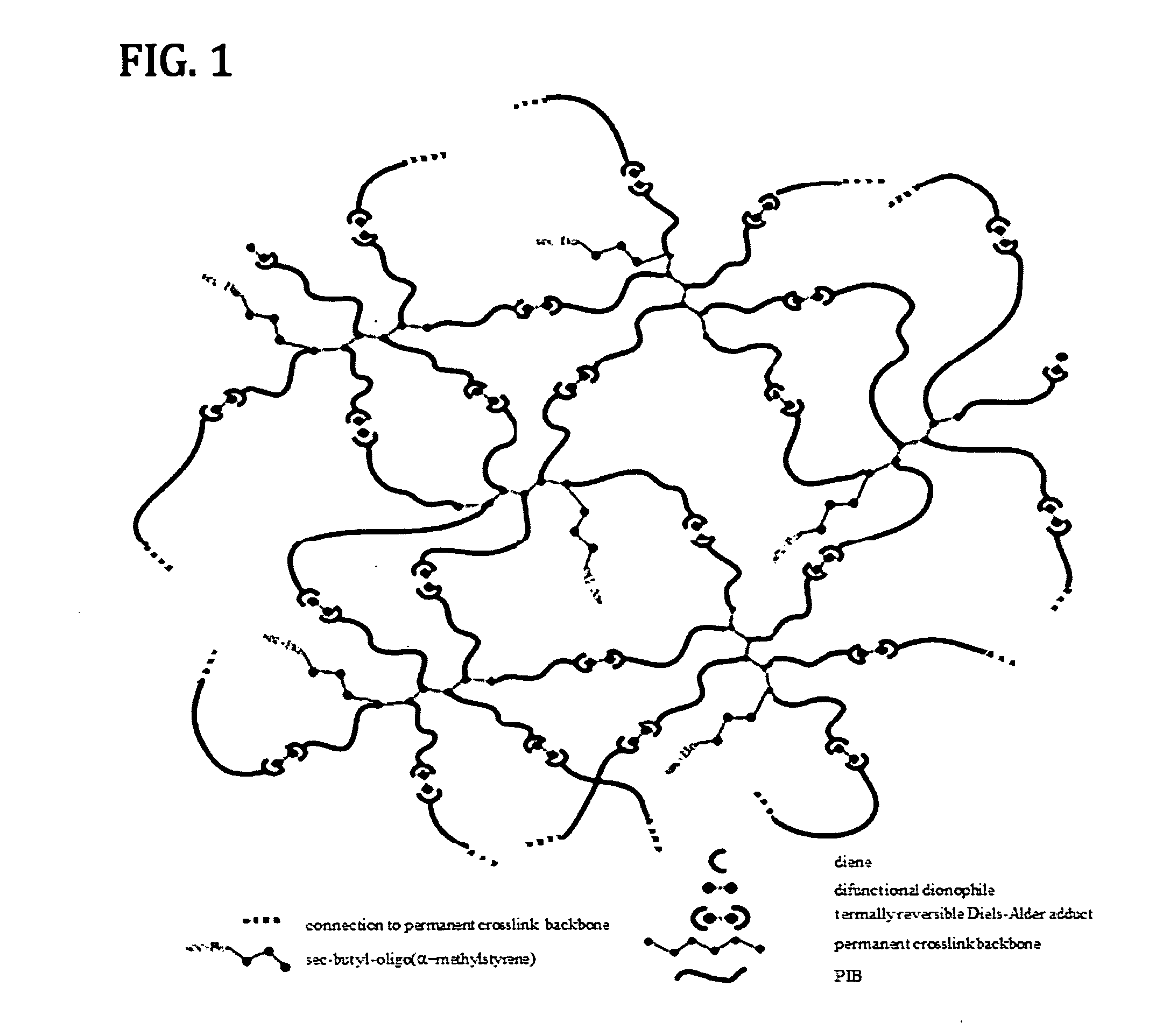
Novel Polyisobutylene-Based Thermoplastic Elastomers
a thermoplastic elastomer, polyisobutylene technology, applied in the field of block copolymers, can solve problems such as inability to be recycled
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
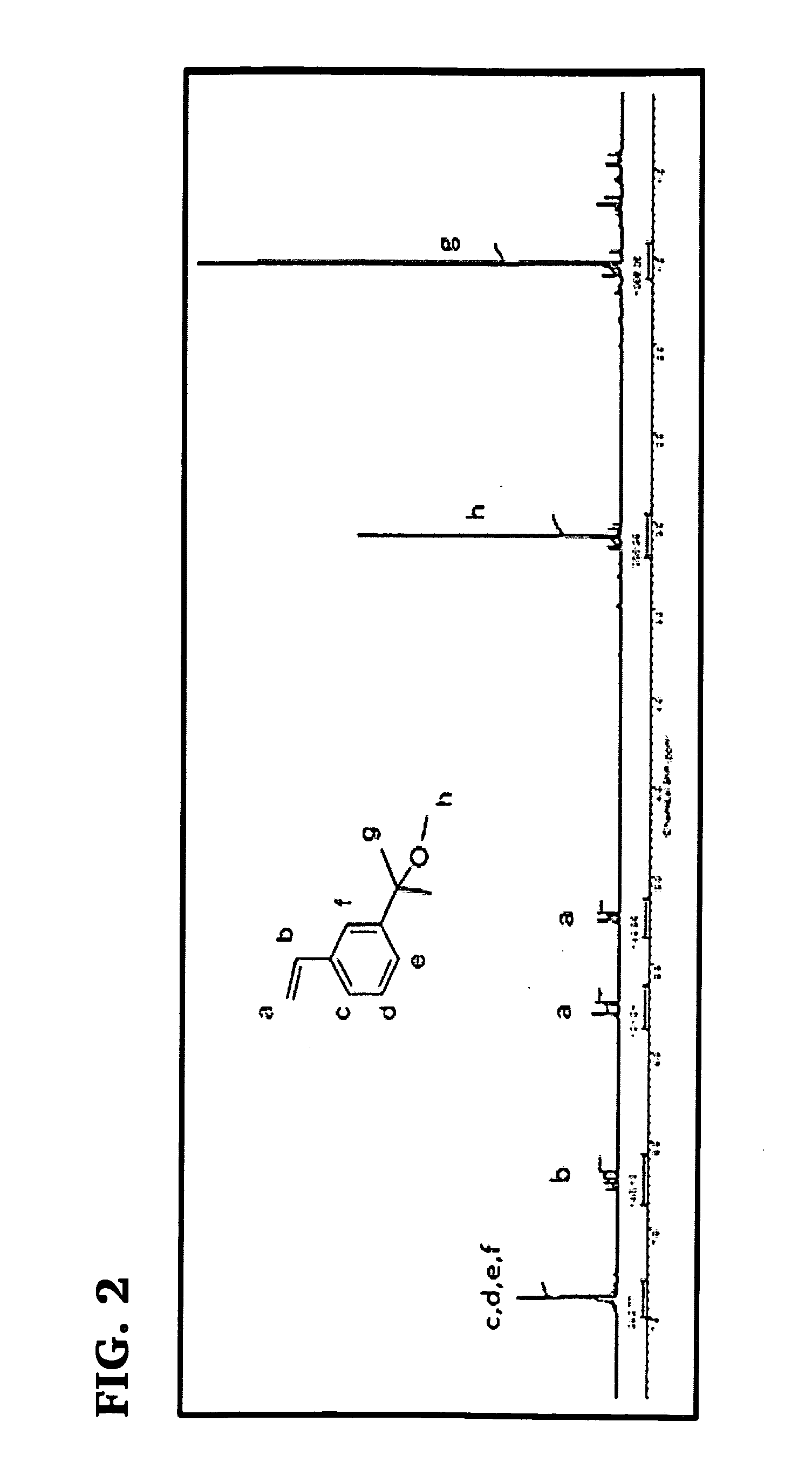
[0082]This example concerns the synthesis of 3-(2-methoxyisopropyl)styrene (i) (MeOiPrSt), a novel monomer suitable for the subsequent preparation of novel graft copolymers carrying PIB arms fitted with furan end groups. The monomer was synthesized using the following reaction scheme:
[0083]A representative procedure for the synthesis of this monomer was as follows: In a 100 mL round bottom flask containing 25 g (0.137 mol) 3-bromostyrene (x) dissolved in dry THF was reacted with 13 g (0.535 mol) magnesium turning in THF at 15° C. for 4 hours under nitrogen atmosphere. Then 30 mL of acetone (xi) was added drop wise to this Grignard reagent (m-vinyl phenyl magnesium bromide) (ii) in 30 minutes at 0° C. and the mixture was stirred overnight at 25° C. The content of the flask was then poured into a beaker with 200 g ice and 5 g NH4Cl. After extraction of the organic phase with diethyl ether three times, the solution was dried over anhydrous MgSO4 overnight. The solids were filtered off ...
example 2
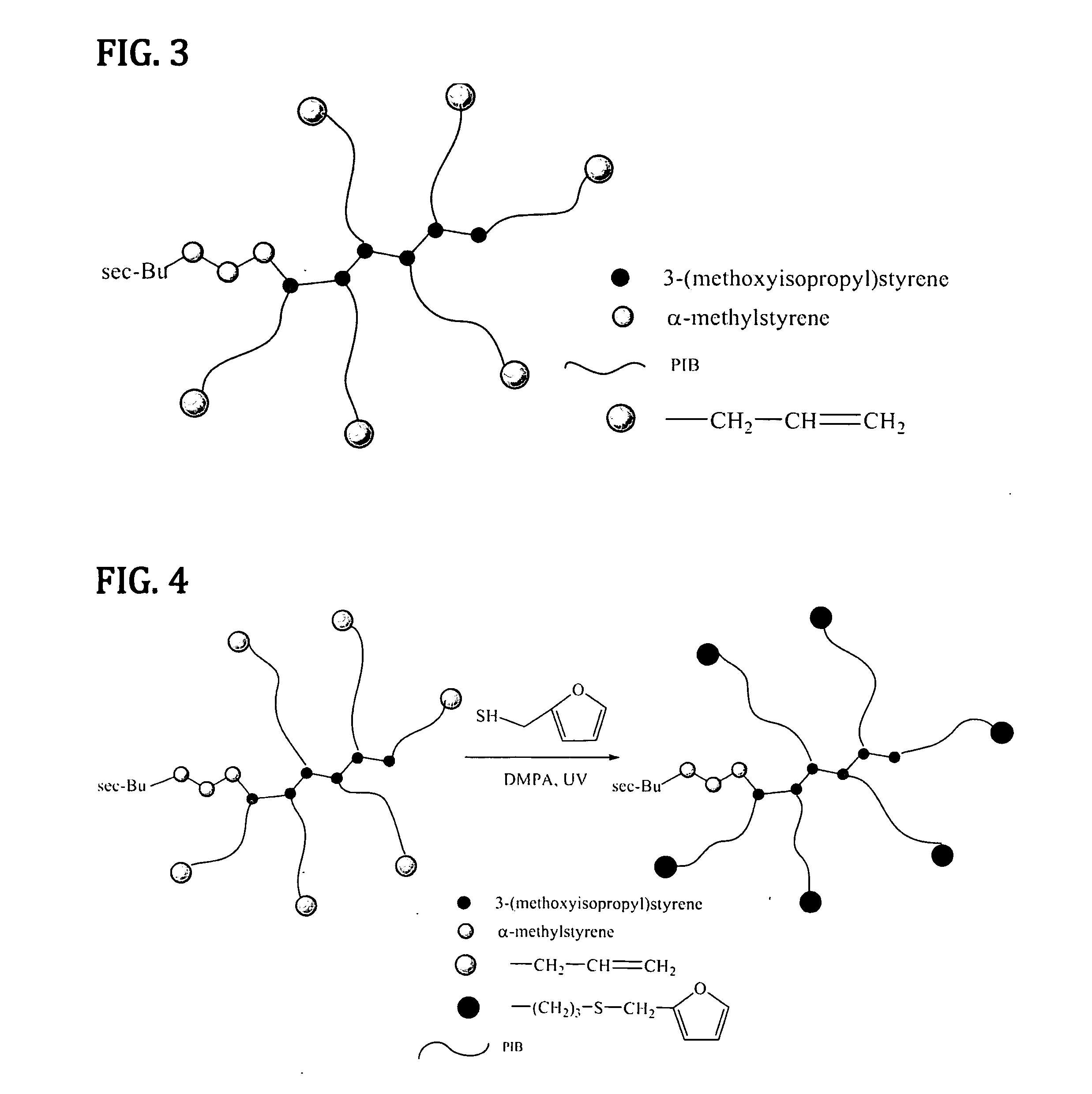
[0085]This example concerns the preparation of a novel hexamer of 3-(2-methoxyisopropyl)styrene (HMeOiPrSt) by anionic polymerization.
[0086]The hexamerization of MeOiPrSt was conducted under high vacuum on a Schlenk line. This monomer was distilled over calcium hydride under reduced pressure and then distilled over dibutylmagnessium under high vacuum conditions just prior to anionic oligomerization. The reaction scheme below outlines the hexamerization. The hexa [3-(2-methoxyisopropyl)styrene] (HMeOiPrSt) was subsequently used to initiate the polymerization of isobutylene.
[0087]The hexa[3-(2-methoxyisopropyl)styrene (HMeOiPrSt) with an α-methylstyrene oligomer section and a sec-butyl head group was synthesized using the following reaction scheme:
[0088]Thus, α-methylstyrene (xii) was distilled over calcium hydride under reduced pressure and then distilled over dibutylmagnessium under high vacuum. To 0.74 mL (5.70×10−4 mol) α-methylstyrene (xii) in 40 mL THF was added drop wise 1.35 m...
example 2a
[0090]An alternative method for the preparation of hexa[3-(methoxyisopropyl)styrene], and in general for the synthesis of oligomeric (10-16 mer) 3-(methoxyisopropyl)styrene, is by initiation with cumyl potassium, whose preparation is well known in anionic polymerization methodology. See Z. Huruska, G. Hurtrez, S. Walter, G. Riess, Polymer, 33, 11, 1992, the disclosure of which is hereby incorporated by reference in its entirety. This cumyl potassium initiator is prepared from cumyl methyl ether and sodium / potassium alloy of suitable composition. See F. Calderara, Z Huruska, G. Hurtrez, T. Nugay, G. Riess, Macromol Chem., 194, 1411, (1993), the disclosure of which is hereby incorporated by reference in its entirety. To a 300 mL round bottom flask having 100 mL freshly cryodistilled THF, previously prepared cumyl potassium is added drop wise under strong stirring until a slightly pink color persists (indication the absence of impurities). Then the required amount of cumyl potassium (5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com