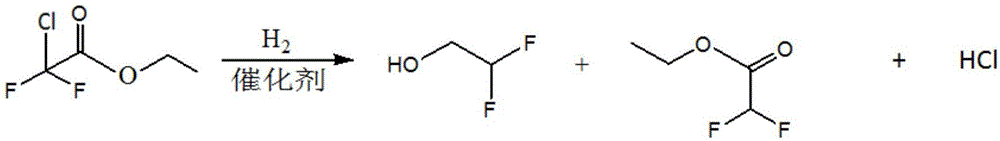
Method for preparing difluoroethanol through catalytic hydrogenation
A technology of difluoroethanol and catalytic hydrogenation, applied in the field of catalytic chemistry, can solve the problems of high equipment cost, complicated operation, large investment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
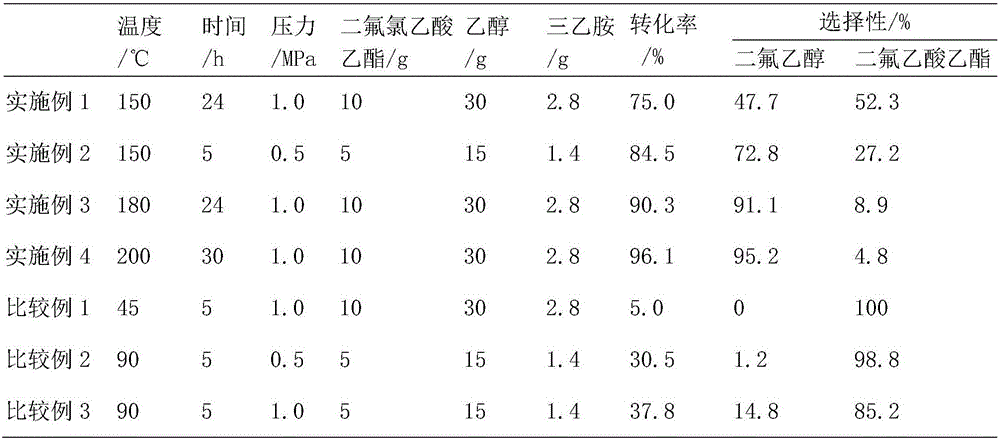
[0030] Take a dry and clean reactor, and add magnets into it. Weigh 0.5g of Pd / C on weighing paper with an electronic balance, weigh 10g of ethyl difluorochloroacetate, 30g of ethanol, and 2.8g of triethylamine with a beaker, add them to the reaction kettle in sequence, and tighten the lid of the reaction kettle. Inflate 4MPa hydrogen with an instrument to check whether the kettle is leaking. If there is no leak, release the gas. Repeat three times, and inflate to 1.0MPa for the last time. Put it in a silicone oil bath at 150°C to react for 24 hours. After the time is up, take it out and cool it until the temperature drops below 60°C, release the gas, unscrew the lid of the reaction kettle, take a sample with a dropper, and conduct chromatographic analysis. The results are shown in Table 1. .
Embodiment 2
[0032] Take a dry and clean reactor, and add magnets into it. Weigh 0.5g of Pd / C on weighing paper with an electronic balance, weigh 5g of ethyl difluorochloroacetate, 15g of ethanol, and 1.4g of triethylamine with a beaker, add them to the reaction kettle in sequence, and tighten the lid of the reaction kettle. Inflate 4MPa hydrogen with an instrument to check whether the kettle is leaking. If there is no leakage, deflate it. Repeat three times, and inflate to 0.5MPa for the last time. Put it in a silicone oil bath at 180°C to react for 5 hours. After the time is up, take it out and cool it until the temperature drops below 60°C, release the gas, unscrew the lid of the reaction kettle, take a sample with a dropper, and conduct chromatographic analysis. The results are shown in Table 1. .
Embodiment 3
[0034] Take a dry and clean reactor, and add magnets into it. Weigh 0.5g of Pd / C on weighing paper with an electronic balance, weigh 10g of ethyl difluorochloroacetate, 30g of ethanol, and 2.8g of triethylamine with a beaker, add them to the reaction kettle in sequence, and tighten the lid of the reaction kettle. Inflate 4MPa hydrogen with an instrument to check whether the kettle is leaking. If there is no leakage, deflate it. Repeat three times, and inflate to 1MPa for the last time. Put it in a silicone oil bath at 180°C to react for 24 hours. After the time is up, take it out and cool it until the temperature drops below 60°C, release the gas, unscrew the lid of the reaction kettle, take a sample with a dropper, and perform chromatographic analysis. The results are shown in Table 1. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com