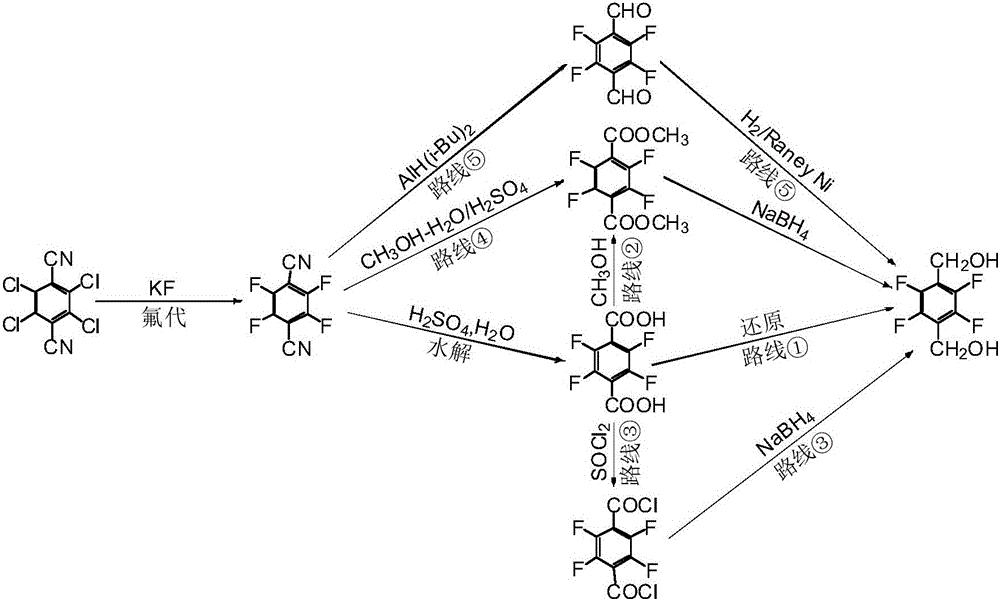
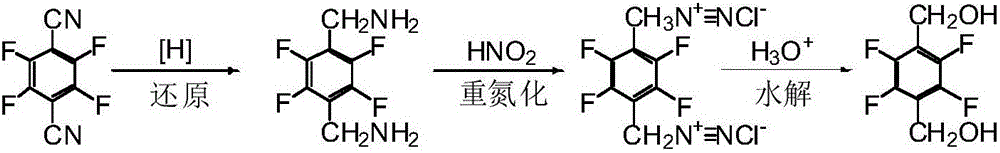
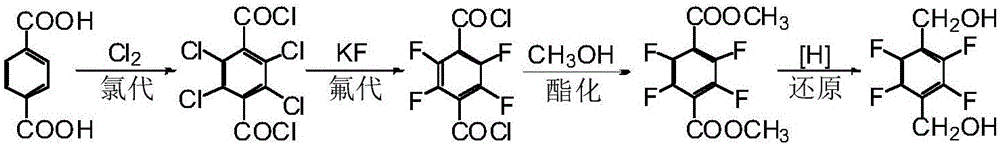
Preparation method of 2,3,5,6-tetrafluorohydrazine-1,4-benzene dimethanol
A technology of benzenedimethanol and tetrafluorobenzene is applied in the field of preparation of intermediate 2,3,5,6-tetrafluoro-1,4-benzenedimethanol, and can solve the problems of complicated operation, many side reactions and long synthesis route and other problems, to achieve the effects of cheap and easy-to-obtain raw materials, reduced product costs, and short synthesis routes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of 2,3,5,6-tetrafluoro-1,4-benzenedimethanol comprises the following steps:
[0045] a. Synthesis of 1,4-bis(trichloromethyl)-2,3,5,6-tetrachlorobenzene (II)
[0046] Add 53.1g p-xylene (I) and 0.5g iron powder to a 250mL four-neck flask equipped with a stirrer, a thermometer, a condenser tube (the end is connected to an exhaust gas absorption device) and a gas inlet tube, raise the temperature to 110°C, and open the The sodium lamp light source at 10cm beside the flask immediately introduces chlorine gas through the airway. Control the temperature at 110-115°C, stir under light, and feed 360g of chlorine gas. After stopping the chlorine gas for 5 minutes, turn off the light source. Then nitrogen gas was introduced under stirring for 15 minutes to drive off residual hydrogen chloride to obtain 201.0 g of compound II.
[0047] b. Synthesis of 1,4-bis(methoxychloromethyl)-2,3,5,6-tetrafluorobenzene(III)
[0048]In a 500mL four-neck flask equipped with ...
Embodiment 2
[0052] In step a, keeping other conditions unchanged, 1.6 g of ferric chloride was used instead of iron powder, the temperature was controlled at 90-95° C. and chlorine gas was introduced into the reaction to obtain 182.5 g of compound II.
[0053] In step b, keeping other conditions unchanged, in a 1000 mL four-necked flask, 600 g of n-butanol was used instead of methanol, 90.5 g of potassium fluoride was added, and the temperature was raised to 120° C. for 6 h to obtain 33.9 g of compound III.
[0054] In step c, keep other conditions unchanged, add 600g n-butanol, 1.0g Pd / C, 33.9g compound III and 79.1g pyridine, react at 115-120°C and hydrogen pressure 5.0MPa to obtain 14.3g compound IV, The purity by HPLC was 96.1%.
Embodiment 3
[0056] In step a, keeping other conditions unchanged, 3.0 g of aluminum trichloride was used instead of iron powder, the temperature was controlled at 50-55° C., and chlorine gas was introduced into the reaction to obtain 171.3 g of compound II.
[0057] In step b, keeping other conditions unchanged, 150 g of ethanol was used instead of methanol, 58.0 g of potassium fluoride, and the temperature was raised to 80° C. for 12 hours to obtain 32.7 g of compound III.
[0058] In step c, keep other conditions unchanged, add 500g methyl tert-butyl ether, 5.0g Raney Ni, 32.7g compound III and 31.6g pyridine, react at 180~185°C and hydrogen pressure 3.0MPa to obtain 13.1g compound IV, the purity detected by HPLC is 96.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com