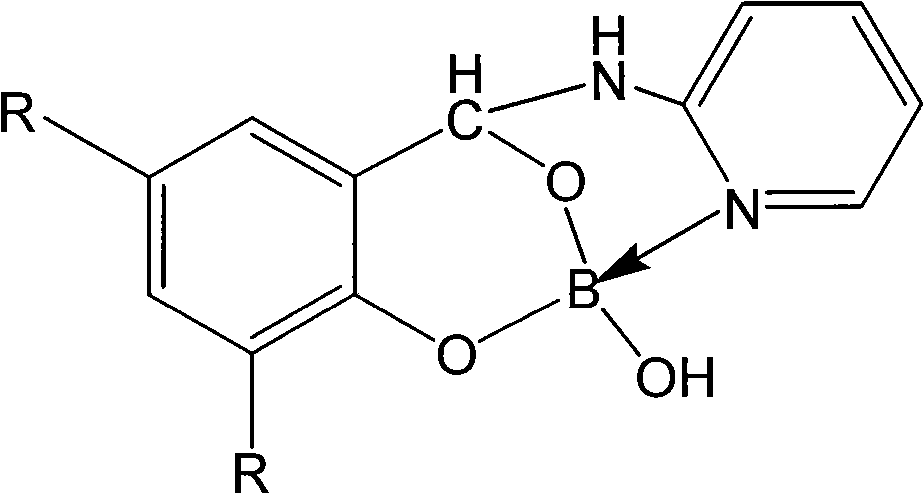
Pyridine-2''amino-2'-hydroxybenzene methanol phenylcarbinol boric acid ester compound and synthetic method thereof
A technology of hydroxybenzyl alcohol and borate esters, which is applied in the field of pyridine-2”-amino-2’-hydroxybenzyl alcohol borate ester compounds and their synthesis, which can solve environmental hazards, high toxicity and release of by-products hydrocyanic acid, The problem of low yield is achieved, and the effect of simple synthesis and abundant raw materials is achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the synthesis of pyridine-2 "-amino-2'-hydroxybenzyl alcohol borate
[0020] Accurately weigh 0.50g (4.1mmol) of salicylaldehyde and dissolve it in 10mL of ethanol, simultaneously weigh 0.39g (4.1mmol) of 2-aminopyridine and dissolve it in 10mL of ethanol, and add the latter dropwise to the ethanol solution of salicylaldehyde , After heating and refluxing under stirring for 5 hours, the ethanol was evaporated under reduced pressure, and recrystallized with 10 mL of ethanol to obtain the product salicylaldehyde acetal 2-aminopyridinium, with a yield of 84.5%.
[0021] Accurately weigh 2.40 g (12.1 mmol) of salicylaldehyde acetal 2-aminopyridinzanthine and dissolve in 20 mL of ethanol-water (V:V=4:1), and then add 0.75 g (12.1 mmol) of boric acid. Control the reflux temperature to 75-80° C., react for 2 hours, filter the obtained solution, extract and place it, and the product is precipitated with a yield of 58.3%.
[0022] verify: 1 H NMR (CD 3 OD) δ6.56...
Embodiment 2
[0024] Example 2: Synthesis of pyridine-2"-amino-3', 5'-dimethyl-2'-hydroxybenzyl borate
[0025] Accurately weigh 0.61g (4.1mmol) of 3,5-dimethyl salicylaldehyde and dissolve it in 10mL of ethanol, and simultaneously weigh 0.39g (4.1mmol) of 2-aminopyridine and dissolve it in 10mL of ethanol, and add the latter dropwise to In the ethanol solution of 3,5-dimethyl salicylaldehyde, after stirring and heating to reflux for 6 hours, the ethanol was evaporated under reduced pressure, and recrystallized with 10mL ethanol to obtain the product 3,5-dimethyl salicylaldehyde acetal 2- Aminopyridinium base, the yield is 61.2%.
[0026] Accurately weigh 2.70 g (12.1 mmol) of 3,5-dimethyl salicylaldehyde acetal 2-aminopyridinzanthine and dissolve it in 20 mL of ethanol-water (V: V=4: 1), then add 0.75 g ( 12.1 mmol) boric acid. Control the reflux temperature to 75-80° C., react for 3 hours, filter the obtained solution, extract, place, and precipitate the product with a yield of 46.3%. ...
Embodiment 3
[0029] Example 3: Synthesis of pyridine-2"-amino-3', 5'-diethyl-2'-hydroxybenzyl alcohol borate
[0030] Accurately weigh 0.73g (4.1mmol) of 3,5-diethyl salicylaldehyde and dissolve it in 10mL of ethanol, simultaneously weigh 0.39g (4.1mmol) of 2-aminopyridine and dissolve it in 10mL of ethanol, and add the latter dropwise to In the ethanol solution of 3,5-diethyl salicylaldehyde, heat and reflux under stirring for 4 hours, evaporate the ethanol under reduced pressure, and recrystallize with 10mL ethanol to obtain the product 3,5-diethyl salicylaldehyde acetal 2- Aminopyridinium base, the yield is 56.2%.
[0031] Accurately weigh 3.08 g (12.1 mmol) of 3,5-diethyl salicylaldehyde acetal 2-aminopyridinzanthine and dissolve it in 20 mL of ethanol-water (V: V=4: 1), then add 0.75 g ( 12.1 mmol) boric acid. Control the temperature at 75-80° C., react for 4 hours, filter the obtained solution, extract and place it, and the product is precipitated with a yield of 44.4%.
[0032] v...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap