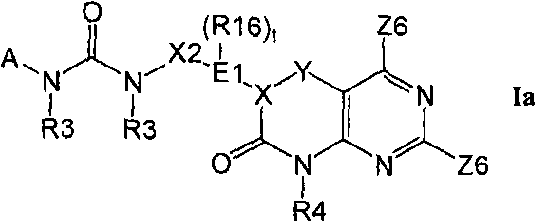
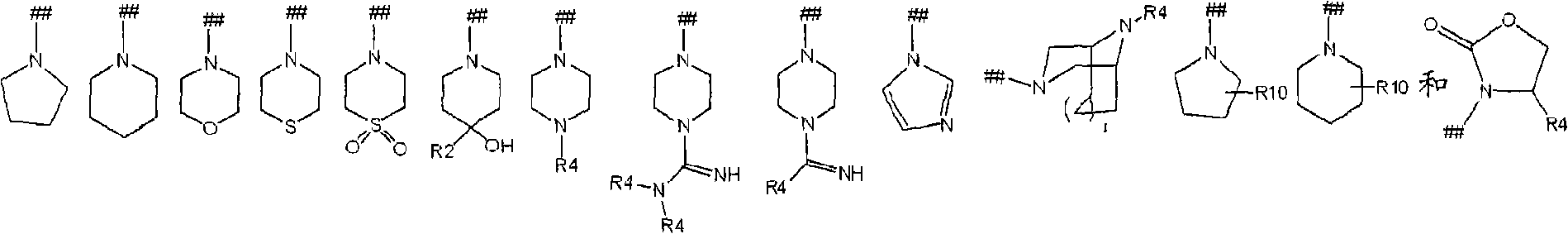
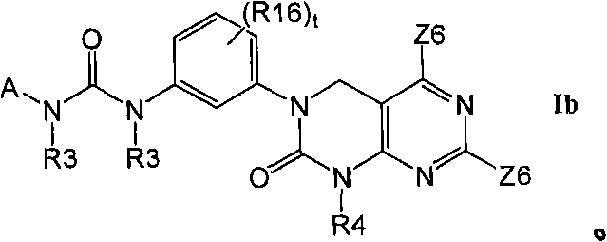
Kinase inhibitors useful for the treatment of proliferative diseases
A sequential, CH2 technology, applied in the field of disease treatment, can solve problems such as out-of-control signal transduction pathways
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A1
[0536] Example A1: Acetic acid (10 mL, 174 mmol) was added to a mixture of Example C2 (10 g, 54.6 mmol) and 4-fluoro-3-nitroaniline (8.5 g, 54.6 mmol) in water (350 mL), and The mixture was stirred overnight at room temperature. The solid was collected by filtration and washed with MeOH (2 x 20 mL) to give 5-((4-fluoro-3-nitrophenylimino)methyl)-N-methyl-2-(methylthio)pyrimidine-4 - Amine (8.0 g, 46% yield) as a yellow solid. 1 H NMR (300MHz, DMSO-d 6 ): δ9.39(m, 1H), 8.71(s, 1H), 8.33(s, 1H), 8.18(m, 1H), 7.80(m, 1H), 7.61(t, J=6.9Hz, 1H) , 3.05 (d, J=3.6Hz, 3H), 2.48 (s, 3H); MS (ESI) m / z: 322.2 (M+H + ).
[0537] to LiAlH at 0°C 4 (1.3g, 34mmol) of anhydrous THF suspension was added in batches to the above 5-((4-fluoro-3-nitrophenylimino)methyl)-N-methyl-2-(methanol) over 20 minutes Thio)pyrimidin-4-amine. After the addition was complete, the mixture was stirred at 0°C for 30 minutes. Aqueous 10% NaOH (2 mL) was added and the resulting precipitate was removed by fil...
Embodiment A2
[0540] Example A2: CH to Example A1 (400mg, 1.3mmol) 2 Cl 2 (5 mL) solution was added 3-chloroperbenzoic acid (mCPBA) (430 mg, 2.5 mmol) in one portion. After stirring for 2 h, the reaction mixture was washed with NaHCO 3 Aqueous solution and NaHSO 3 The aqueous solution was quenched. The organic layer was separated, washed with brine, and dried (Na 2 SO 4 ), concentrated in vacuo. The crude product was dissolved in DMSO (2 mL) and treated with ammonia in dioxane (2M, 30 mL, 60 mmol). The mixture was stirred overnight at room temperature. The reaction was concentrated under reduced pressure and the residue was purified by silica gel chromatography to give 7-amino-3-(3-amino-4-fluorophenyl)-1-methyl-3,4-dihydropyrimido[4 ,5-d]pyrimidin-2(1H)-one (276 mg, 77% yield) as a yellow solid. 1 H NMR (300MHz, DMSO-d 6 ): δ7.88(s, 1H), 6.96(dd, J=8.4, 6.6Hz, 1H), 6.68(dd, J=6.3, 2.1Hz, 1H), 6.52(br s, 2H), 6.44(m , 1H), 5.18(s, 2H), 4.48(s, 2H), 3.19(s, 3H); MS (ESI) m / z: 289....
Embodiment A3
[0541] Example A3: Example A1 (1.0 g, 3.1 mmol), mCPBA (1.1 g, 6.3 mmol) and methylamine were combined by the process of Example A2 to give 3-(3-amino-4-fluorophenyl)- 1-Methyl-7-(methylamino)-3,4-dihydropyrimido[4,5-d]pyrimidin-2(1H)-one (370 mg, 39% yield) as a yellow solid. 1 H NMR (400MHz, DMSO-d 6 ): δ7.93(s, 1H), 6.99-6.94(m, 2H), 6.69(d, J=8.4Hz, 1H), 6.44(m, 1H), 5.18(s, 2H), 4.50(s, 2H), 3.23(s, 3H), 2.78(d, J=4.0Hz, 3H); MS (ESI) m / z: (M+H + )303.2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com