Cell culture system, method for the production thereof and use in preclinical testing
A cell culture, pre-clinical technology, applied in the field of cell culture system, can solve the problem of increased requirements and achieve the effect of avoiding abnormal over-concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
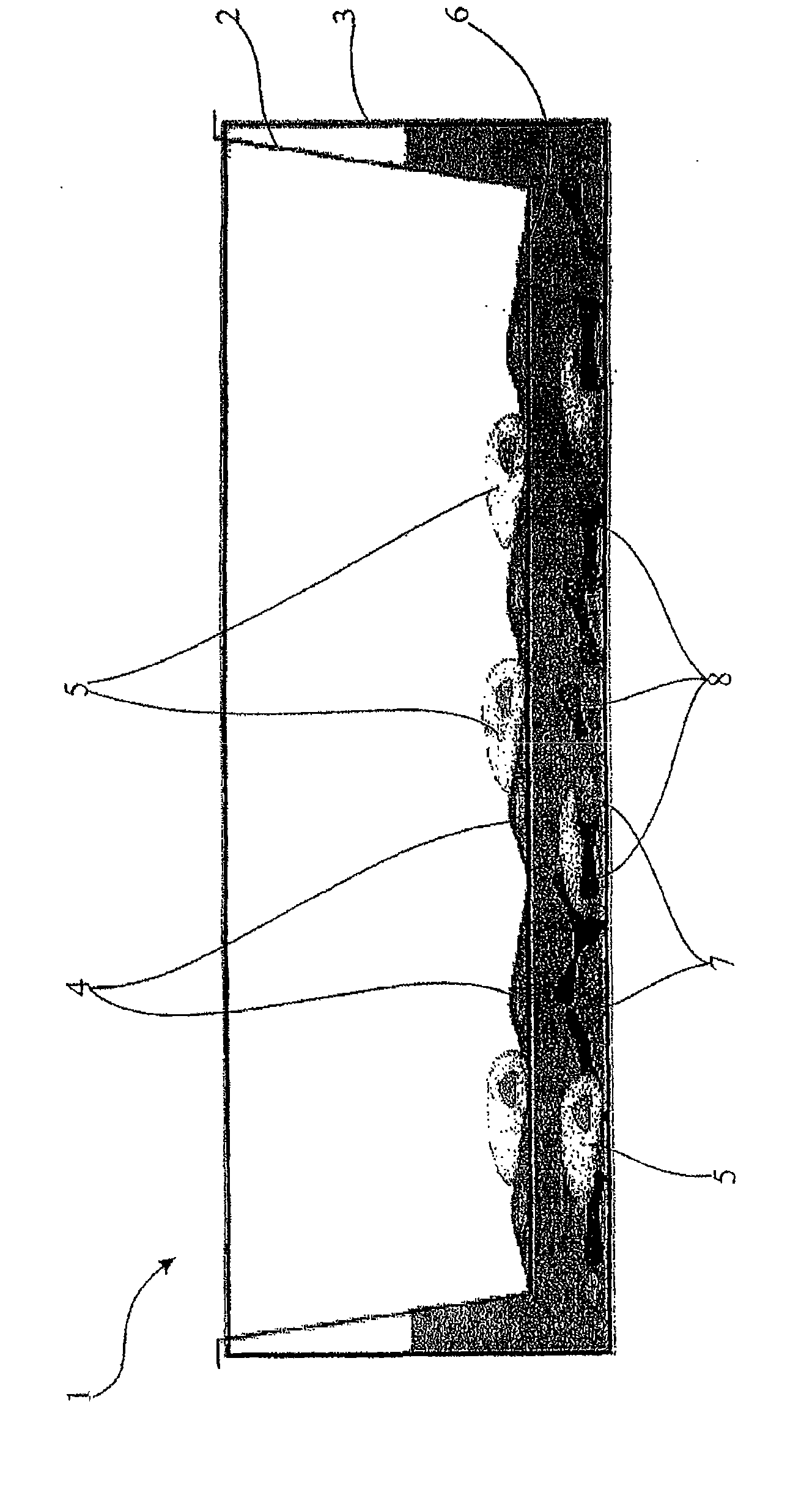
[0026] In preferred embodiments, the tissue cells of the first compartment are adherent. Tissue cells preferably adhere to the surface of the separation layer. The separation layer can be precoated with a suitable substance. Substances can be, for example, proteins, especially extracellular matrix proteins. The separation layer may be coated with collagen, laminin, tenascin, etc., for example.
[0027] The tissue cells of the first compartment are advantageously pre-cultured on the surface of the separating layer. In particular the invention provides tissue cells to cover at least part, preferably the entire, compartment. Tissue cells may cover the separation layer, in particular in the form of a layer, preferably in the form of a monolayer.
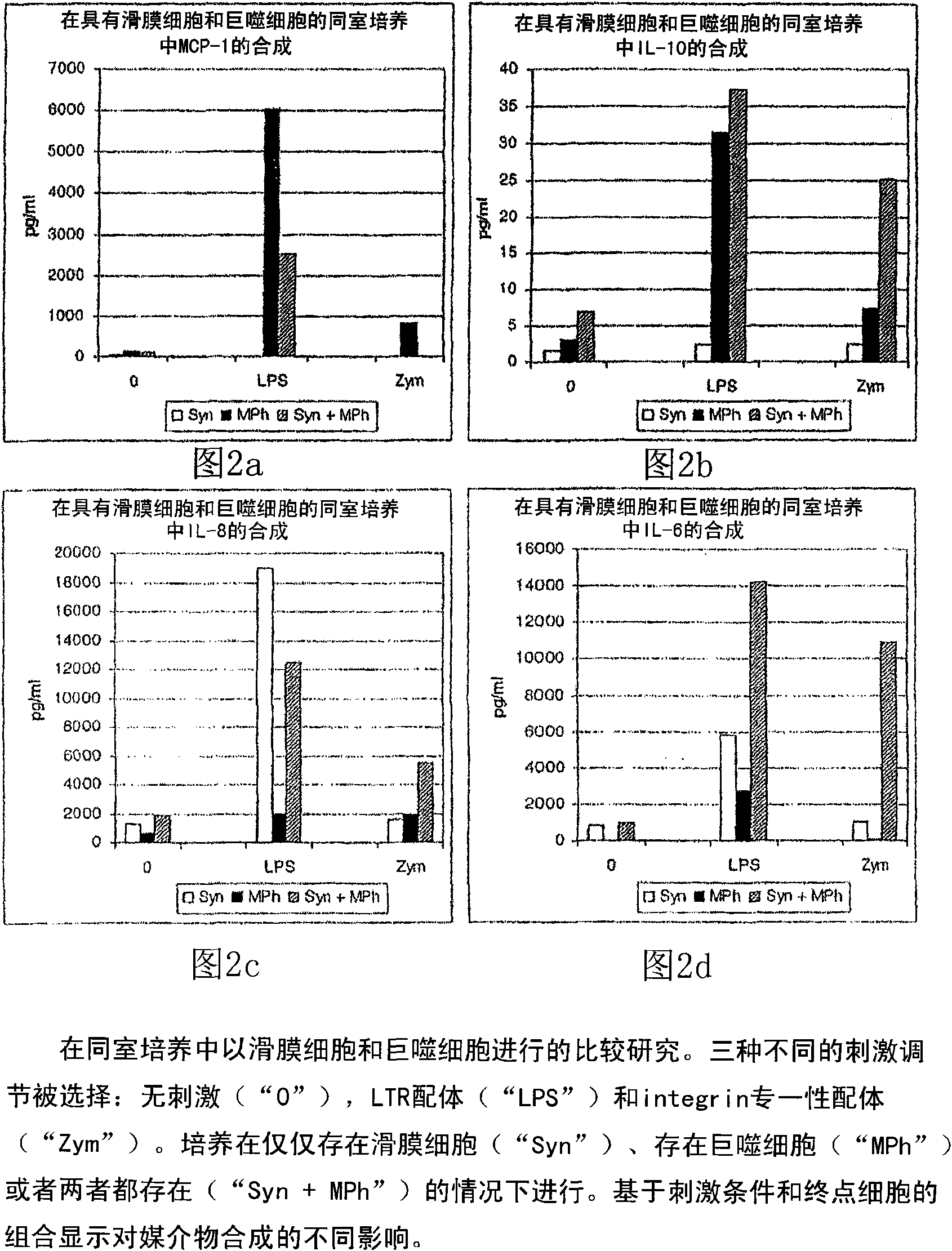
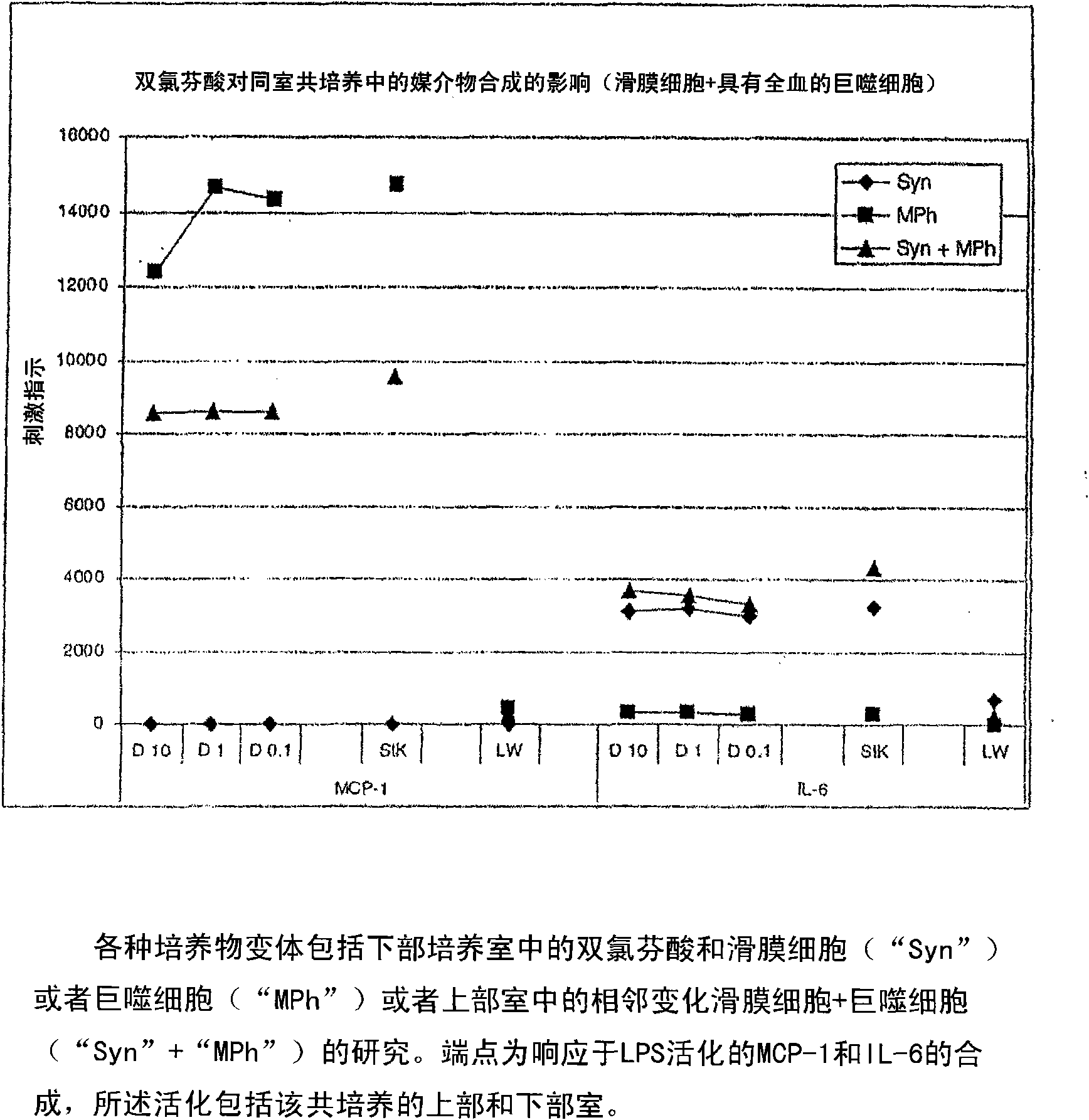
[0028] In a particularly preferred embodiment, the immune cells are phagocytic immune cells, especially monocytes and / or macrophages. Monocytes and macrophages represent immunoregulatory switching centers in the immune system. They a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com