Loop-mediated isothermal amplification technology-based Listeria monocytogenes rapid diagnostic kit and testing method thereof
A technology for rapid diagnosis of Listeria, applied in the direction of microbe-based methods, biochemical equipment and methods, microbiological determination/inspection, etc., can solve the problem of unseen gene rapid diagnostic kits for detecting Listeria monocytogenes , to achieve significant color difference, high specificity, and rapid amplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 kit
[0040] (1) Synthesize oligodeoxynucleic acid primers by DNA synthesizer according to the following sequence:
[0041] Outer primer F3: ACAAGACTTCACCAATCCA, as shown in SEQ ID NO: 1;
[0042] Outer primer B3: GTCTTTTAAGTGGAGTAAACCTT, as shown in SEQ ID NO: 2;
[0043] Internal primer FIP: TAAGTCTCTTTGCAATTGACCGACTTTTACGTGTACACAGAAAAAGCG, as shown in SEQ ID NO: 3;
[0044] Internal primer BIP: CCTGTGCCAAAGCATTTTTACATTTTTTAGGCAAGTCATCTTGTTCG, as shown in SEQ ID NO: 4;
[0045] (2) Purchasing DNA polymerase: Bst DNA polymerase is placed in the container.
[0046] (3) Preparation of reaction solution: the reaction solution contains 2mmol / LdNTP, 25mmol / L Tris-Cl, 12.5mmol / L potassium chloride, 12.5mmol / L ammonium sulfate, 10mmol / L magnesium sulfate, 0.125% by volume TritonX-100, 1mol / L betaine, 2 mol / L each of the inner primers FIP / BIP and 0.25 mol / L each of the outer primers F3 / B3 were placed in the container.
[0047] (4) Prepare ...
Embodiment 2
[0058] The preparation of embodiment 2 kit
[0059] The formula of the reaction solution is: the reaction solution contains 1.8mmol / LdNTP, 20mmol / LTris-Cl, 10mmol / L potassium chloride, 10mmol / L ammonium sulfate, 8mmol / L magnesium sulfate, 0.1vol%TritonX-100, 0.8mol / L Betaine, 1.6 mol / L of inner primer FIP / BIP and 0.2 mol / L of outer primer F3 / B3.
[0060] The formula of the sample pretreatment liquid is: the sample pretreatment liquid contains 10 mmol / L Tris-HCl (pH 8.0), 1 mmol / L EDTA and 1 volume % Triton X-100.
[0061] Others are the same as embodiment 1.
Embodiment 3
[0062] Example 3 Application of Listeria monocytogenes Gene Rapid Diagnostic Kit
[0063] 1 Materials and methods
[0064] 1.1 Materials
[0065] 1.1.1 Strains
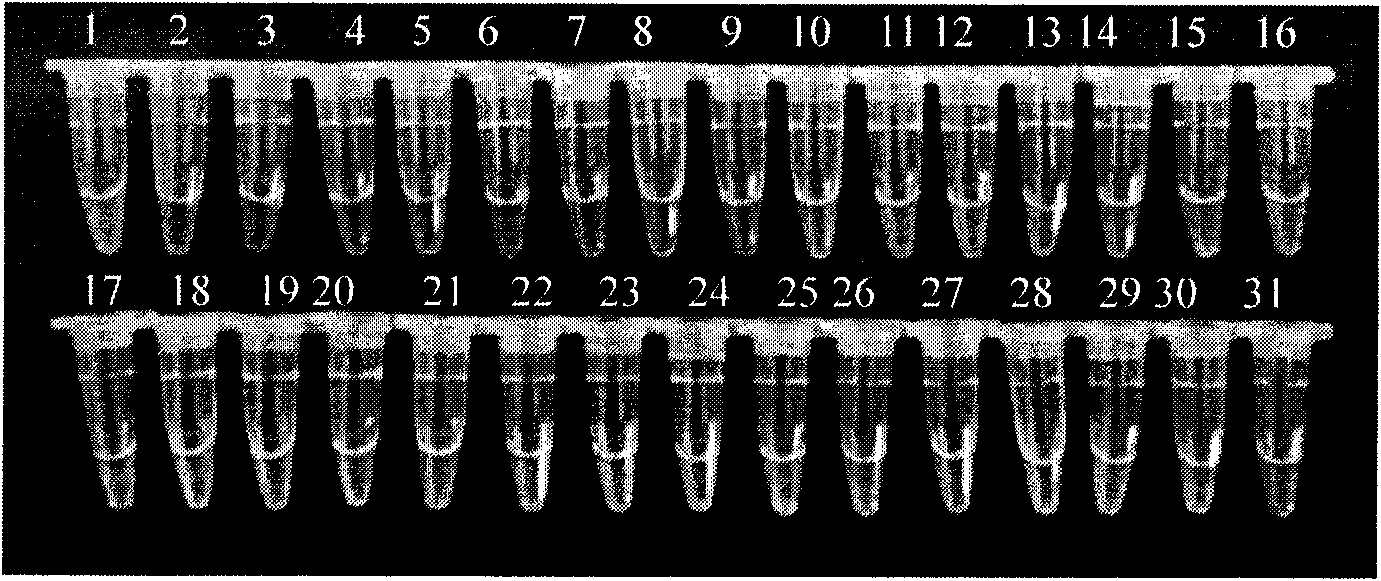
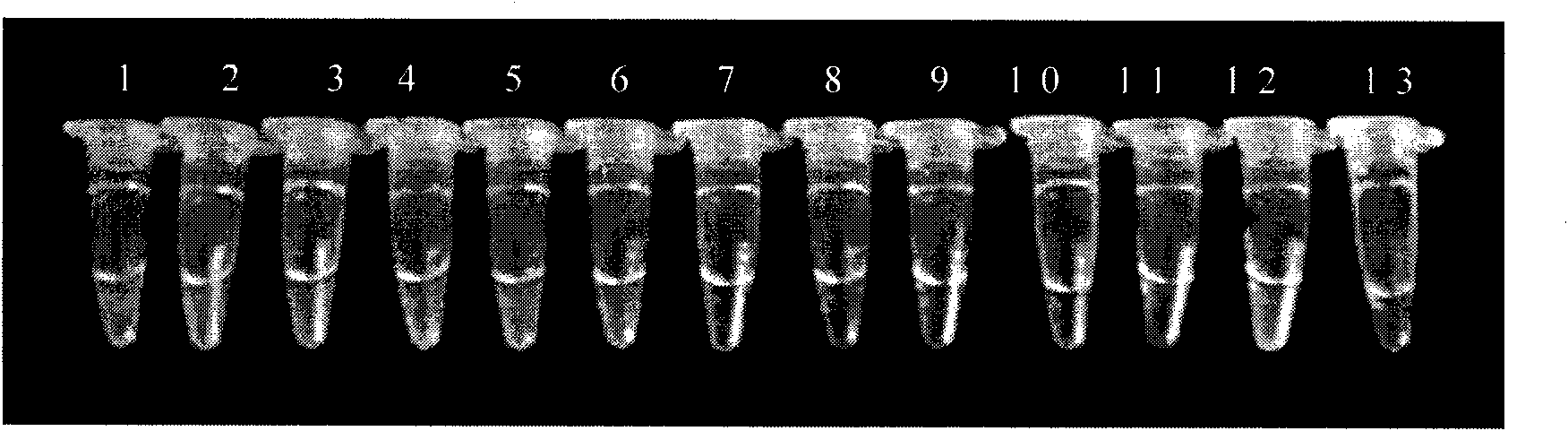
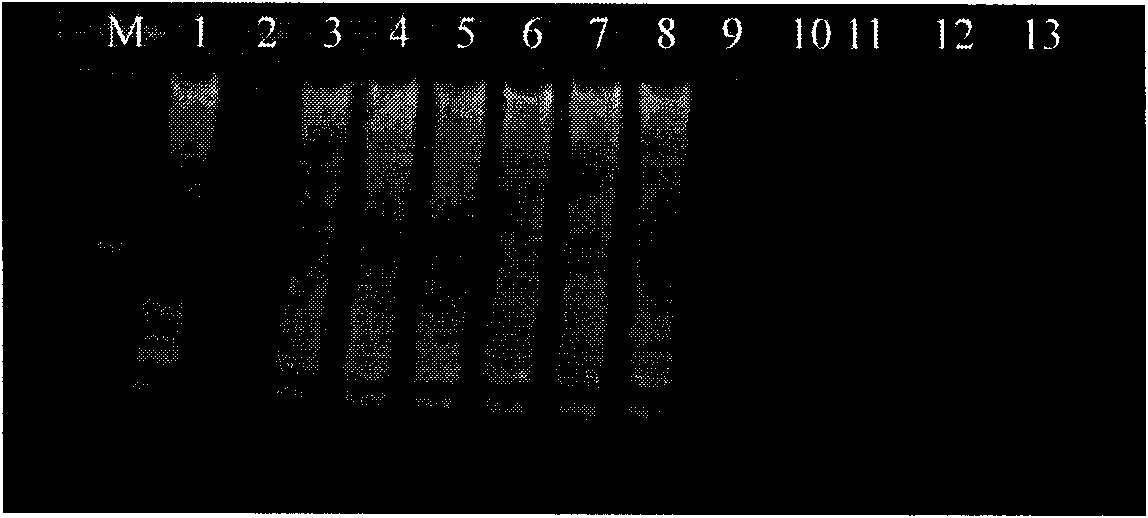
[0066] There are 28 bacterial strains used by the company, mainly from the American Standard Biological Collection Center, clinical isolates and environmental isolates. See Table 1 for details.
[0067] Table 1 Names and sources of strains
[0068] strain source
Strain and serial number
American Standard Biological Collection
Center (ATCC)
Listeria monocytogenes (7466), Listeria innocuci (33090),
Listeria ovis (19119), Listeria wales (35897),
Listeria gasseri (25401);
Clinical isolates
Derived from 20 strains of Listeria monocytogenes in the test sample;
other strains
Salmonella, Escherichia coli O157, Staphylococcus aureus each 1 strain.
[0069] 1.1.2 Main instruments and reagents
[0070] 1.2 Identification of isolated strains
[0071] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com