Medicament for treating chronic prostatitis, and preparation method thereof
A chronic prostatitis and drug technology, which is applied in the directions of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients, etc., can solve the problems affecting the physical and mental health and life of patients, affecting the work and rest of patients, etc. Fewer side effects and clear pharmacological effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
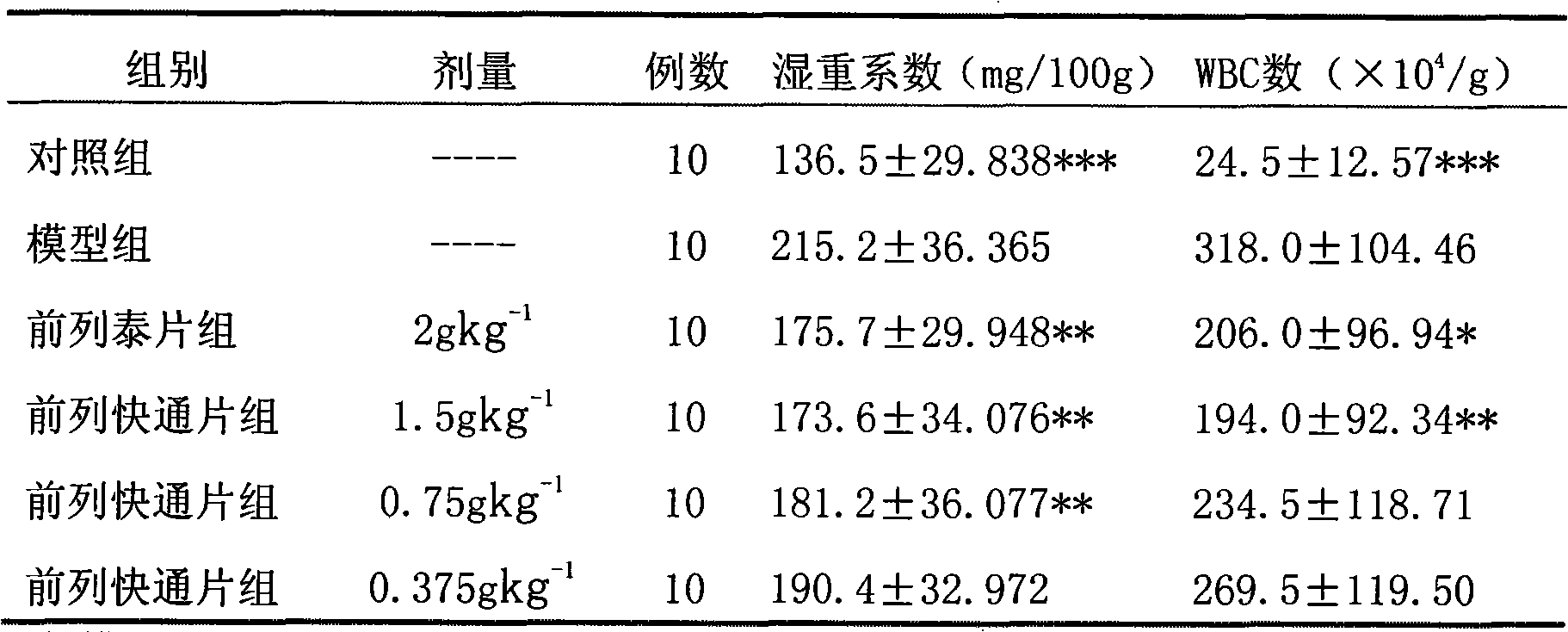
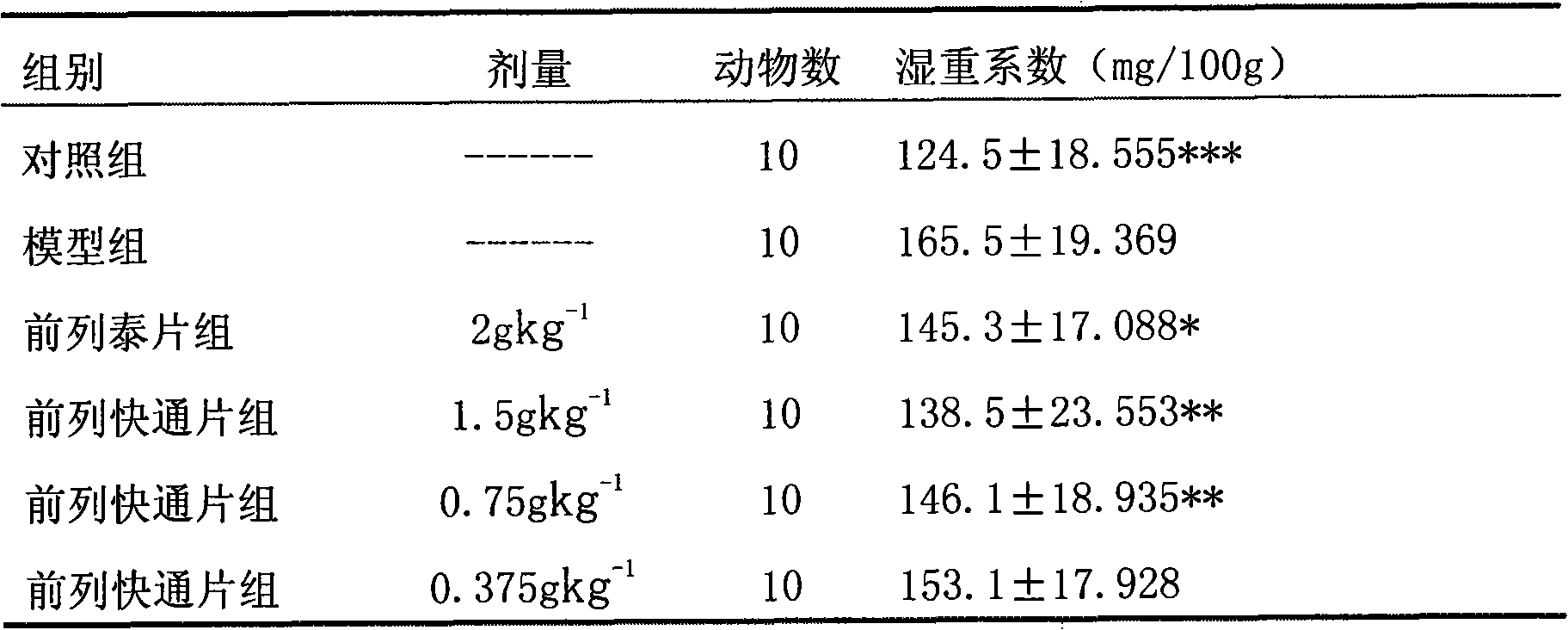
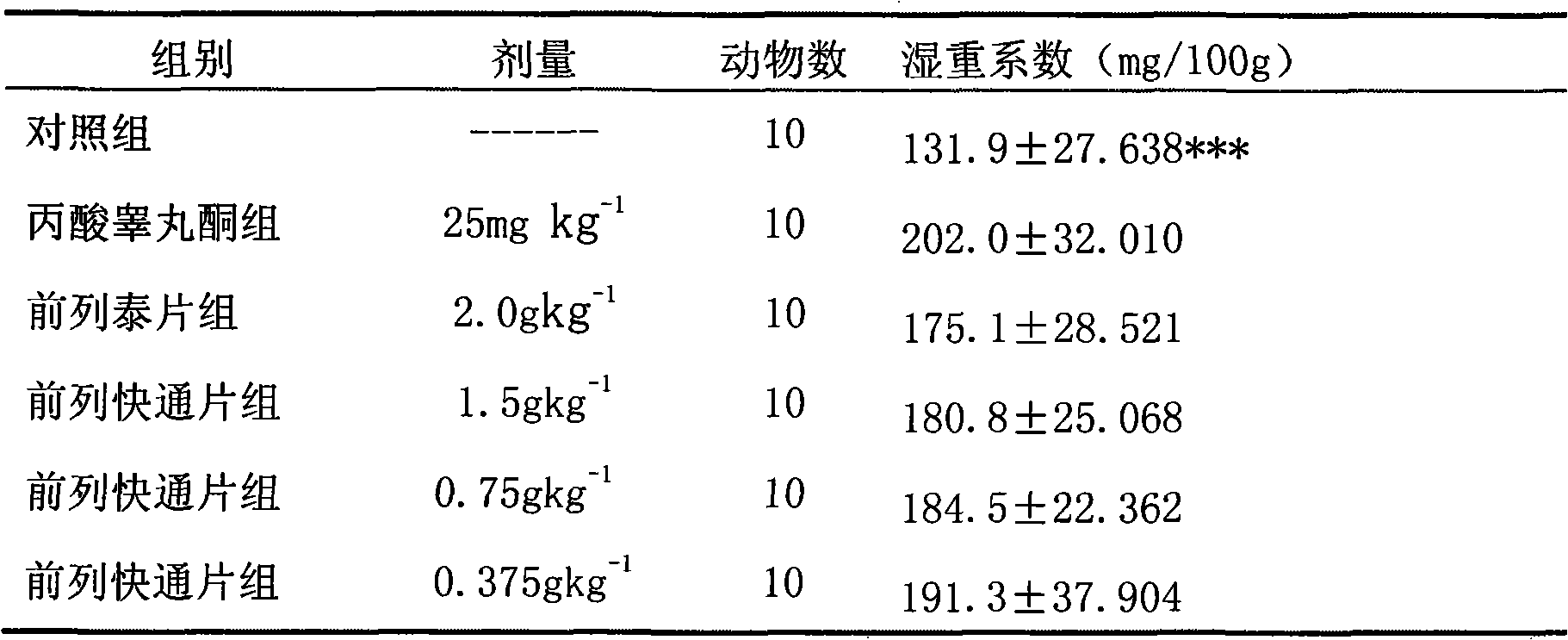
Image
Examples
Embodiment 1
[0144] The capsule preparation of embodiment 1 medicine of the present invention
[0145] Polygonum cuspidatum 450g, Smilax tuckahoe 300g, turtle shell 300g,
[0146] Radix Paeoniae Rubra 150g, Tribulus Terrestris 300g, Epimedium 300g,
[0147] Astragalus 300g, medlar 200g, Morinda officinalis 200g.
[0148] The preparation method of pharmaceutical active component of the present invention is as follows:
[0149] a) Take 1 / 10 amount of Polygonum cuspidatum and crush it into fine powder;
[0150] b) Add 5 times the amount of 80% ethanol to the remaining Polygonum cuspidatum and Morinda officinalis, reflux and extract twice, each time for 1 hour, combine the extracts, filter, and recover the ethanol from the filtrate under reduced pressure and concentrate it to a thick product with a relative density of 1.30 at 80°C paste;
[0151] c) Crush the turtle shell to 20 mesh, add 7 times the amount of water and decoct 3 times, 1 hour each time, combine the decoction, filter, and co...
Embodiment 2
[0155] The tablet preparation of embodiment 2 medicines of the present invention
[0156] Polygonum cuspidatum 500g, Smilax tuckahoe 350g, turtle shell 350g, red peony 200g, tribulus terrestris 350g, epimedium 350g, astragalus 350g, medlar 250g, Morinda officinalis 250g.
[0157] The preparation method of pharmaceutical active component of the present invention is as follows:
[0158] a) Take 1 / 10 amount of Polygonum cuspidatum and crush it into fine powder;
[0159] b) Add 6 times the amount of 80% ethanol to the remaining Polygonum cuspidatum and Morinda officinalis, reflux and extract twice, each time for 2 hours, combine the extracts, filter, recover the ethanol from the filtrate under reduced pressure and concentrate it to a thick product with a relative density of 1.33 at 80°C paste;
[0160] c) The turtle shell was crushed to 30 mesh, added 8 times the amount of water and decocted for 3 times, 2 hours each time, combined the decoction, filtered, and the filtrate was c...
Embodiment 3
[0164] The preparation of embodiment 3 granules of the present invention
[0165] Polygonum cuspidatum 550g, Smilax tuckahoe 400g, turtle shell 400g,
[0166] Radix Paeoniae Rubra 250g, Tribulus Terrestris 400g, Epimedium 400g,
[0167] Astragalus 400g, medlar 300g, Morinda officinalis 300g.
[0168] The preparation method of pharmaceutical active component of the present invention is as follows:
[0169] a) Take 1 / 10 amount of Polygonum cuspidatum and crush it into fine powder;
[0170] b) Add 7 times the amount of 80% ethanol to the remaining Polygonum cuspidatum and Morinda officinalis, reflux and extract twice, each time for 3 hours, combine the extracts, filter, and recover the ethanol from the filtrate under reduced pressure and concentrate it to a thick product with a relative density of 1.35 at 80°C paste;
[0171] c) Pulverize the turtle shell to 40 mesh, add 9 times the amount of water to decoct 3 times, 3 hours each time, combine the decoction, filter, and conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com