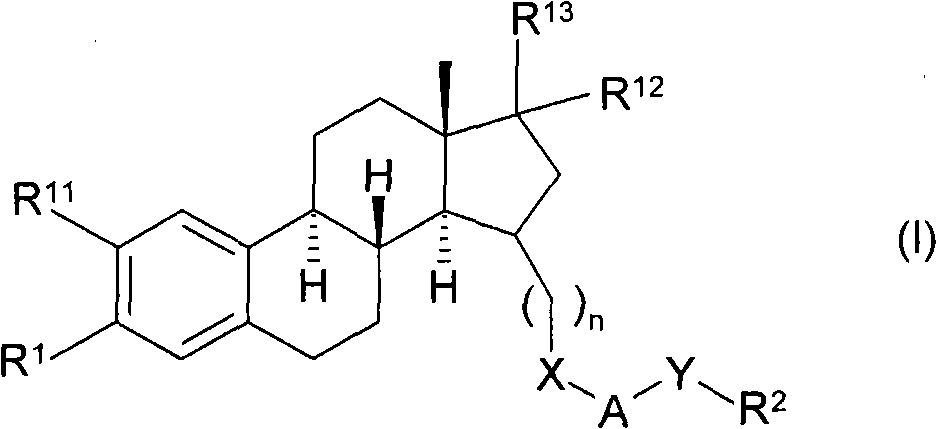
Substituted estratrien derivatives as 17beta hsd inhibitors
A technology of substituents and compounds, applied in the field of substituted estratriene derivatives, can solve problems such as no display
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0940] Example 1: 15α-(4-morpholin-4-yl-4-oxobutyl)-17-oxoestro-1(10), 2,4-triene-3-carboxamide
[0941]
[0942] Example 1 According to the method described in Scheme 6B and 6C(I) and from 3-hydroxy-15α-(4-morpholin-4-yl-4-oxo-butyl)-estr-1,3, 5(10)-triene-17-ones were prepared, the detailed synthesis of which is described in International Patent Application WO2005 / 047303 (Example 40 therein) and can generally follow Route 1, Route 14 or 15 and Scheme Ia or the reaction of Ib to achieve.
[0943] 15α-(4-morpholin-4-yl-4-oxo-butyl)-3-trifluoromethanesulfonate-estro -1,3,5(10)-trien-17-one
[0944] Dissolve 5.4g of 3-hydroxy-15α-(4-morpholin-4-yl-4-oxo-butyl)-estro-1,3,5(10)-triene-17-one in 120ml without water in DCM. 10,72 g of 2,6-lutidine were added slowly. 6.76 g of trifluoromethanesulfonic anhydride in 40 ml of anhydrous DCM was added slowly at 0°C. After stirring at 0 °C for 30 min, the reaction mixture was kept at room temperature overnight and washed with H...
Embodiment 2
[0955] Example 2: 15α-(4-morpholin-4-yl-4-oxobutyl)-17-oxoestro-1(10), 2,4-triene-3-carboxylic acid
[0956]
[0957] Example 2 According to the method described in scheme 6B and from 3-hydroxy-15α-(4-morpholin-4-yl-4-oxo-butyl)-estra-1,3,5(10)- Trien-17-ones start to be prepared, the detailed synthesis of which is described in International Patent Application WO2005 / 047303 (Example 40 therein) and can generally follow the reactions of Route 1, Route 14 or 15 and Scheme Ia or Ib to accomplish. The detailed synthesis method of Example 2 has been described during the synthesis of Example 1.
[0958] 13 C NMR (126MHz, chloroform-d)
[0959] δppm 15.6 (q, 1C) 23.7 (t, 1C) 26.2 (t, 1C) 27.6 (t, 1C) 29.5 (t, 1C) 31.6 (t, 1C) 33.1 (t, 1C) 36.2 (d, 1C) 36.3 (t, 1C) 39.2 (d, 1C) 42.1 (t, 1C) 43.1 (t, 1C) 45.0 (d, 1C) 46.1 (t, 1C) 50.3 (s, 1C) 54.8 (d, 1C) 66.6 (t , 1C) 67.0 (t, 1C) 126.0 (d, 1C) 127.0 (s, 1C) 127.5 (d, 1C) 130.6 (d, 1C) 136.5 (s, 1C) 145.7 (s, 1C) 170.8 (s, 1C)...
Embodiment 3
[0962] Example 3: 15β-{3-[(5-methyl-1,3-thiazol-2-yl)amino]-3-oxopropyl}-17-oxoestro-1(10), 2 , 4-triene-3-carboxamide
[0963]
[0964] Example 3 was prepared according to the methods described in Schemes 6B and 6C(I) and for Example 1 herein, from 3-(3-hydroxy-17-oxo-estro-1,3,5 Starting with (10)-triene-15β-yl)-N-(5-methyl-thiazol-2-yl)-propionamide, its detailed synthesis is described in International Patent Application WO2005 / 047303 (Example 329A ) and can generally be achieved by following the reactions of Scheme 1, Scheme 13 and Scheme Ia or Ib herein.
[0965] 3-(17-Oxo-3-trifluoromethanesulfonate-estra-1,3,5(10)-triene-15β- base)-N-(5-methyl-thiazol-2-yl)-propionamide
[0966] This intermediate was obtained from 3-(3-hydroxy-17-oxo-estro-1,3,5(10)-trien-15β-yl)-N-(5 -Methyl-thiazol-2-yl)-propionamide Preparation.
[0967] 15β-{3-[(5-methyl-1,3-thiazol-2-yl)amino]-3-oxopropyl}-17-oxo Estro-1(10), 2,4-triene-3-carboxylic acid methyl ester
[0968] 2 g of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com