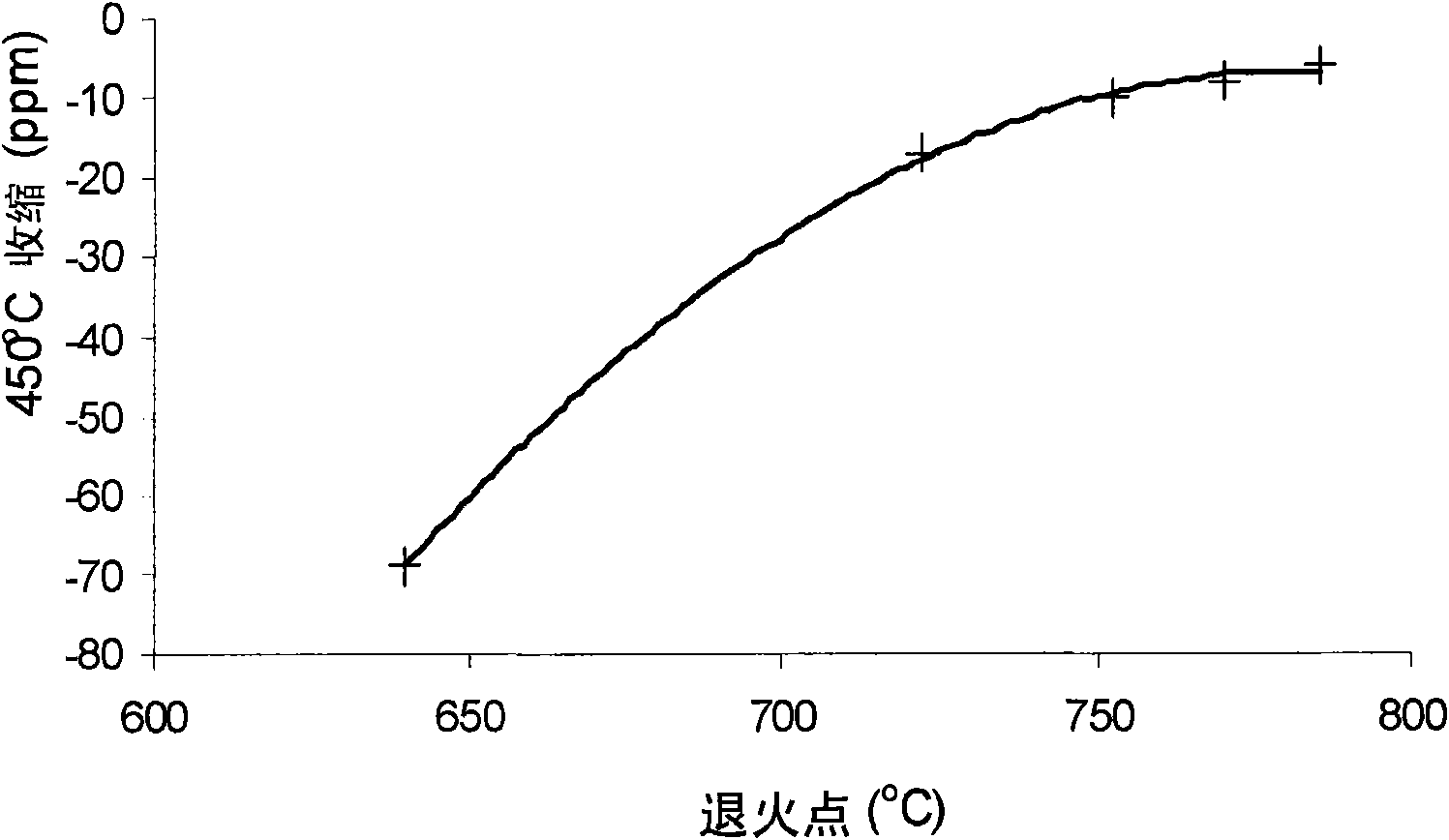
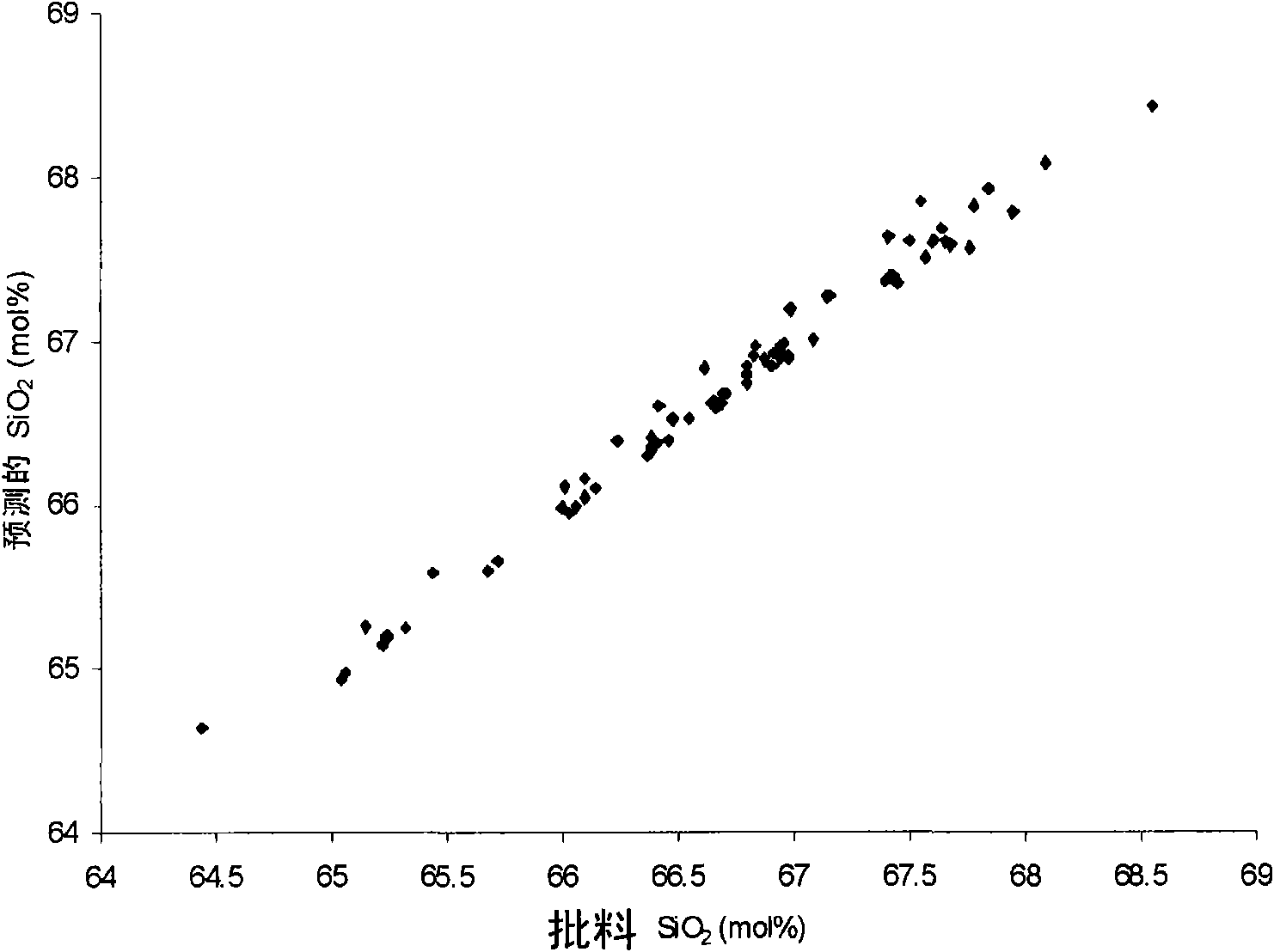
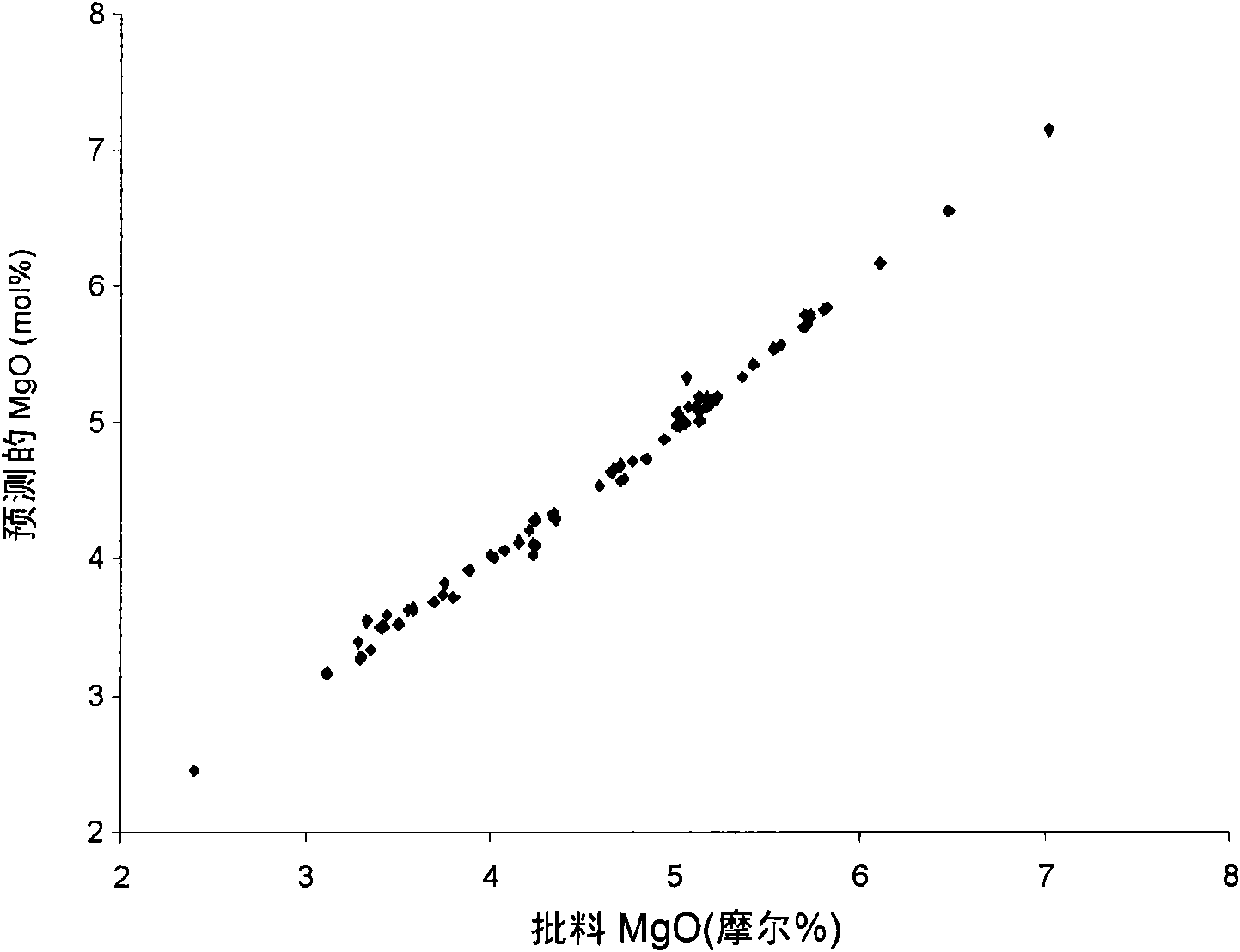
Boroalumino silicate glasses
A glass and alkali-free glass technology, applied in glass forming, glass forming, glass manufacturing equipment, etc., can solve the problems of difficulty in obtaining thermodynamically stable glass plates, high cost, low production rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Example 1 and the following Examples 2 and 3 are intended to describe how the present invention is prepared. These embodiments are not meant to be restricted in any way; rather, the embodiments are provided to facilitate those skilled in the art to have the right to use the method considered by the applicant and use this method of consideration. If appropriate, the content described herein is further developed to optimize the glass of the present invention for specific applications.
[0124] Several surprising findings led to the present invention.
[0125] The first is that when the glass is designed without B 2 O 3 In terms of composition, the glass with the best combination of liquidus viscosity, melting temperature, and annealing point tends to have a similar ratio of SiO 2 , Al 2 O 3 , MgO, CaO and SrO. In order to understand this, it is the easiest to consider an example of a glass. For further discussion, the glass also introduces various required components, glass 6...
Embodiment 2
[0142] The examples show the process of testing the composition of the glass to determine whether it is particularly suitable for the melting process. Use [MgO] pred And [SiO 2 ] pred To optimize the glass used in the melting process of the present invention.
[0143] Considering the glass discussed in Example 1, 67SiO 2 -9B 2 O 3 -11Al 2 O 3 -3MgO-7CaO-3SrO. As mentioned above, a simple calculation shows that the oxide composition of the glass is in the following range:
[0144] 64≤SiO 2 ≤68.2;
[0145] 11≤Al 2 O 3 ≤13.5;
[0146] 5≤B 2 O 3 ≤9;
[0147] 2≤MgO≤9;
[0148] 3≤CaO≤9; and
[0149] 1≤SrO≤5.
[0150] Moreover, a simple calculation shows that the expression (MgO+CaO+SrO) / Al 2 O 3 =(3+7+3) / 11=1.18; (SrO+CaO) / Al 2 O 3 =(3+7) / 11=0.91; and CaO / (CaO+SrO)=7 / (7+3)=0.7, in the following range:
[0151] 1.05≤(MgO+CaO+SrO) / Al 2 O 3 ≤1.45;
[0152] 0.67≤(SrO+CaO) / Al 2 O 3 ≤0.92; and
[0153] 0.45≤CaO / (CaO+SrO)≤0.95.
[0154] Using the expression given above, calculate R as follows ...
Embodiment 3
[0171] Example 3 illustrates the process of producing a glass composition using the relationship described in Example 1.
[0172] The process includes the following steps:
[0173] (1) Select the annealing point target;
[0174] (2) Choose R 0 And S 0 Test value
[0175] (3) Use R 0 And S 0 Calculation [MgO] 0 ;
[0176] (4) Use [MgO] 0 Calculation [SiO 2 ] 0 As the target value of MgO;
[0177] (5) Use R 0 , S 0 , [MgO] 0 And [SiO 2 ] 0 Calculation [Al 2 O 3 ] 0 , [CaO] 0 And [SrO] 0 ;
[0178] (6) Calculate B 2 O 3 And use it to calculate SiO 2 , B 2 O 3 , Al 2 O 3 , The renormalized concentration of MgO and CaO; and
[0179] (7) Compare the result with the required density and CTE target value, if necessary, repeat steps 1-6 with new input parameters.
[0180] In order to carry out these steps, we use three empirical formulas describing the dependence of composition on annealing point, thermal expansion coefficient and density, namely:
[0181] Annealing point = 828.3+3.1Al 2 O...
PUM
| Property | Measurement | Unit |
|---|---|---|
| liquidus temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com