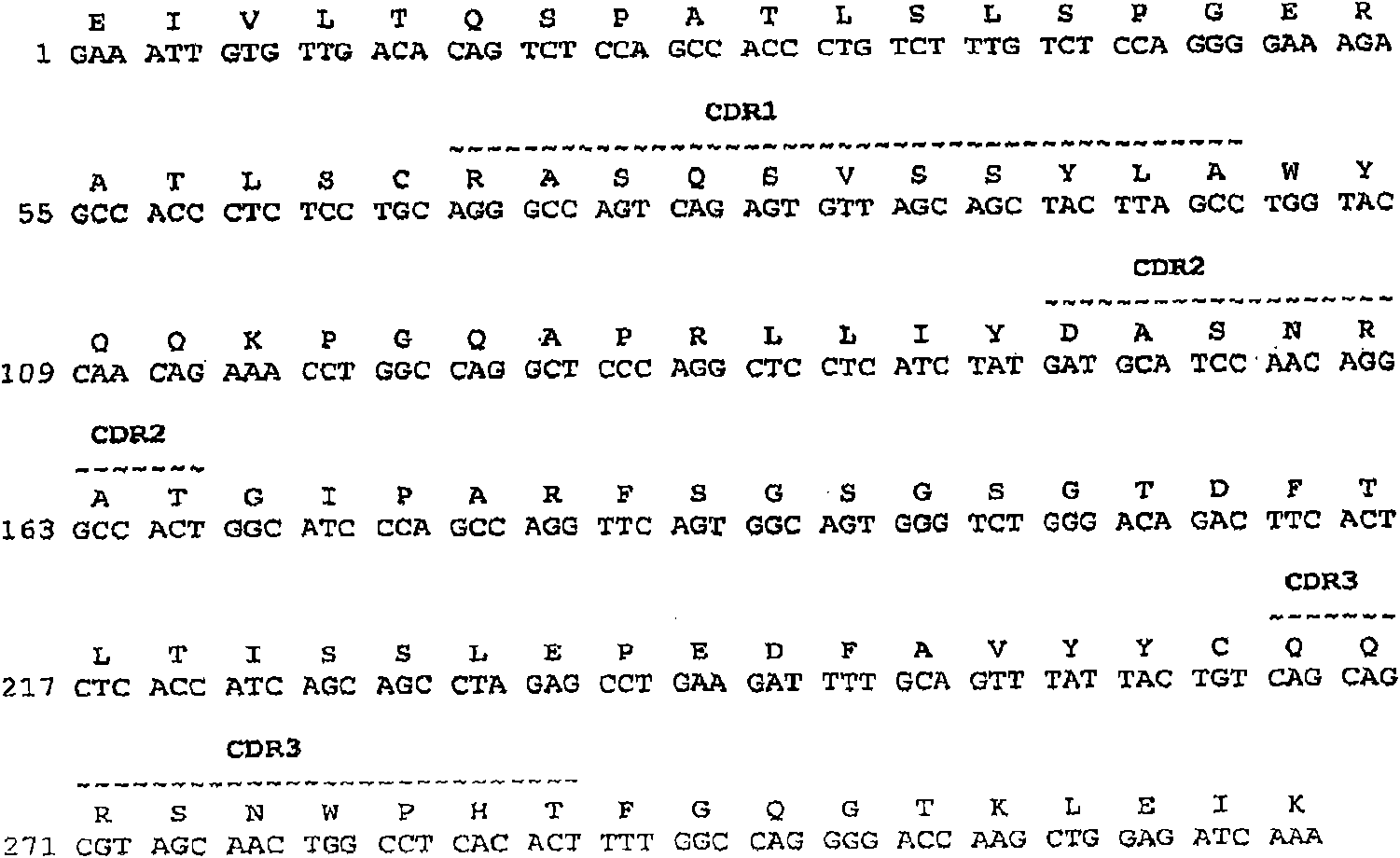Antibodies to bone morphogenic proteins and receptors therefor and methods for their use
An antibody, antibody fragment technology, applied in the fields of immunology and molecular biology, can solve problems such as the inability to prevent bone formation and spinal fusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0474] Human monoclonal anti-BMP2, BMP4, BMPR1A, BMPR1B, ACTR1 and BMPR2 Antibody production
[0475] This example discloses methodology for generating human monoclonal antibodies that specifically bind human BMP2, BMP4, BMPR1A, BMPR1B, ACTR1, and BMPR2.
[0476] antigen
[0477] Mice were immunized with recombinant human BMP2, BMP4, BMPR1A, BMPR1B, ACTR1 and / or BMPR2. Specifically, mice were immunized with commercially available recombinant human BMP2 or BMP4. Human recombinant BMP-2 was obtained from R&D Systems, Inc. (Catalog No. 355-BM / CF, Lot.-MSA10605H) or Medtronic, Inc. (Lot.-M115006AAJ). Human recombinant BMP4 was obtained from R&D Systems, Inc. (Catalog No. 31-BP / CF, Lots BEM186051 and BEM316071 and MSA10605H). Lyophilized antigen was reconstituted according to the manufacturer's instructions and stored at -20°C.
[0478] transgenic mouse HuMAb and KM
[0479] Fully human monoclonal antibodies against BMP2, BMP4, BMPR1A, BMPR1B, ACTR1 and BMPR2 can...
Embodiment 2
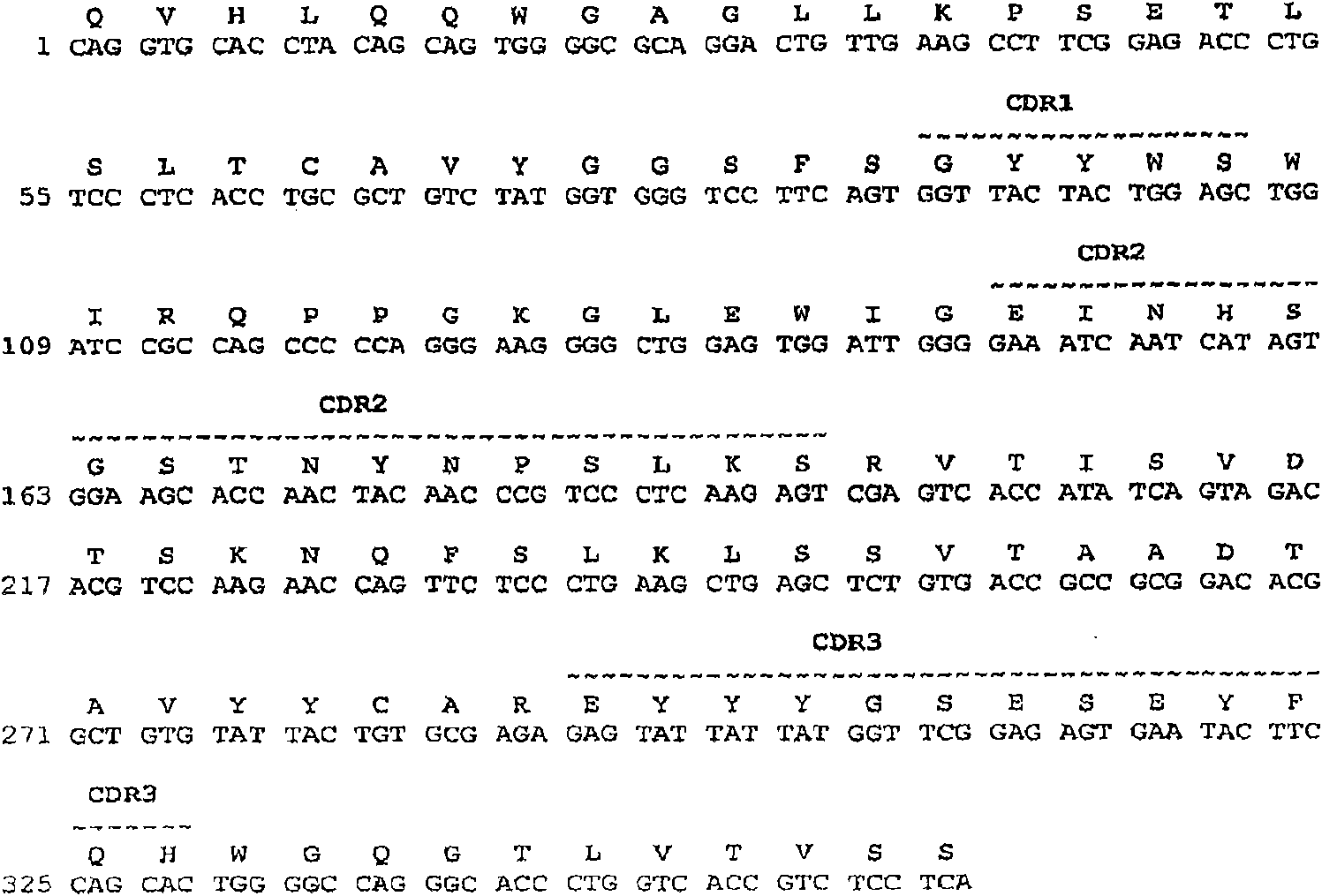
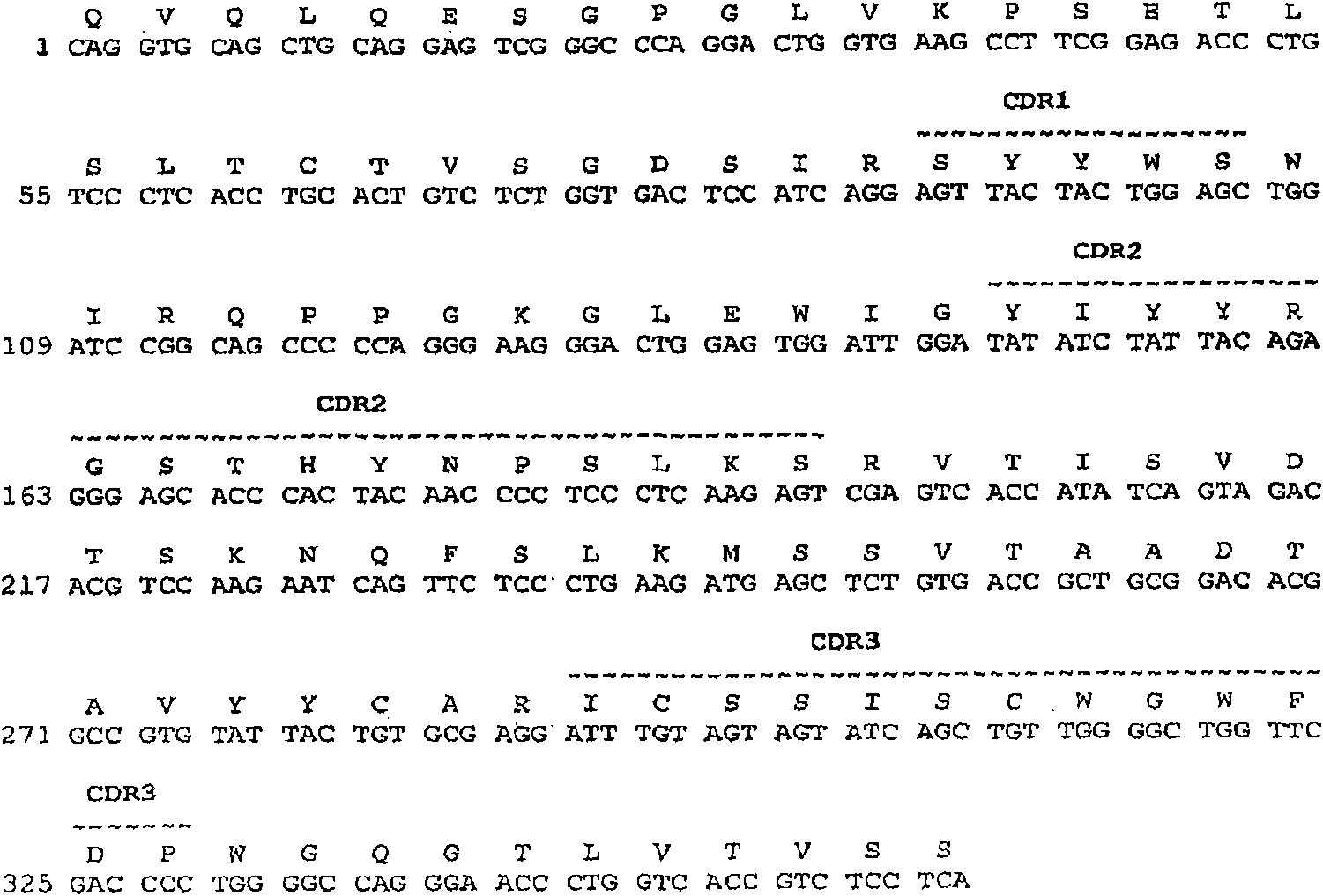
[0492] Structural Characterization of Human Monoclonal Antibodies
[0493] This example discloses the structural characteristics of human monoclonal antibodies that specifically bind to BMP2 and BMP4. Specifically, the structures of anti-BMP2 / 4 monoclonal antibodies 6H4, 11F2, 12E3, 1F6, 10F6, 10H6, 16b7, 7D6, 8B3, 33F7 and 15F3 are disclosed in this example.
[0494] The monoclonal antibodies obtained by the methodology of Example 1 can be obtained from anti-BMP2, anti-BMP4, anti-BMPR1A, anti-BMPR1B, anti-ACTR1 and / or anti-BMPR2 hybridomas, respectively, by standard PCR techniques The cDNA sequences encoding the heavy and light chain variable regions were obtained and sequenced by standard DNA sequencing techniques.
[0495] The cDNA sequences encoding the heavy and light chain variable regions of the 6H4, 11F2 and 12E3 monoclonal antibodies, respectively, were obtained from the 6H4, 11F2 and 12E3 hybridomas by standard PCR techniques and sequenced by standard DNA sequenci...
Embodiment 3
[0509] Anti-BMP2, Anti-BMP4, Anti-BMPR1A, Anti-BMPR1B, Anti-ACTR1 Characterization of Binding Specificity of Anti-BMPR2 Monoclonal Antibodies
[0510] This example discloses the methodology used to make the following comparisons: Anti-BMP2, anti-BMP4, anti-BMPR1A, anti-BMPR1B, anti-ACTR1 and / or anti-BMPR2 antibodies were compared by ELISA and Western blot assays with Antigen binding specificity was examined by immunopurified antigen binding, or by comparing the binding of the antibody to BMP2 / 4 in tissue using immunohistochemistry.
[0511] Recombinant His-tagged or myc-tagged antigens were coated overnight on plates and then tested for binding to human monoclonal antibodies generated by the methodology disclosed in Example 1. Perform standard ELISA procedures. Anti-BMP2, anti-BMP4, anti-BMPR1A, anti-BMPR1B, anti-ACTR1 and / or anti-BMPR2 human monoclonal antibodies were applied at a concentration of 1 μg / ml and titrated down in serial dilutions of 1:2. A goat anti-human I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com