Recombinant modified vaccinia virus ankara (mva)-based vaccine for the avian flu
A vaccinia virus, improved technology, applied in the direction of viruses, antiviral agents, viral antigen components, etc., can solve the problems of unsuitability for preparation and application to humans, and achieve the effect of reducing the dose of vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
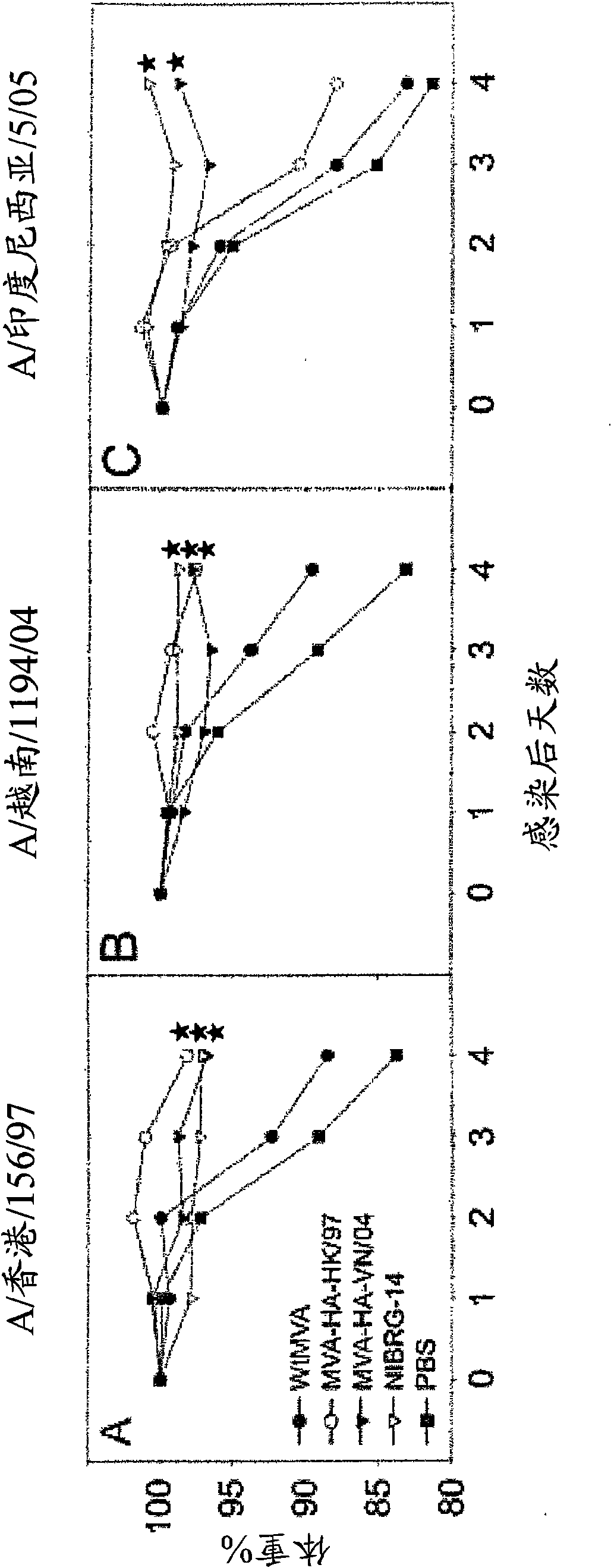
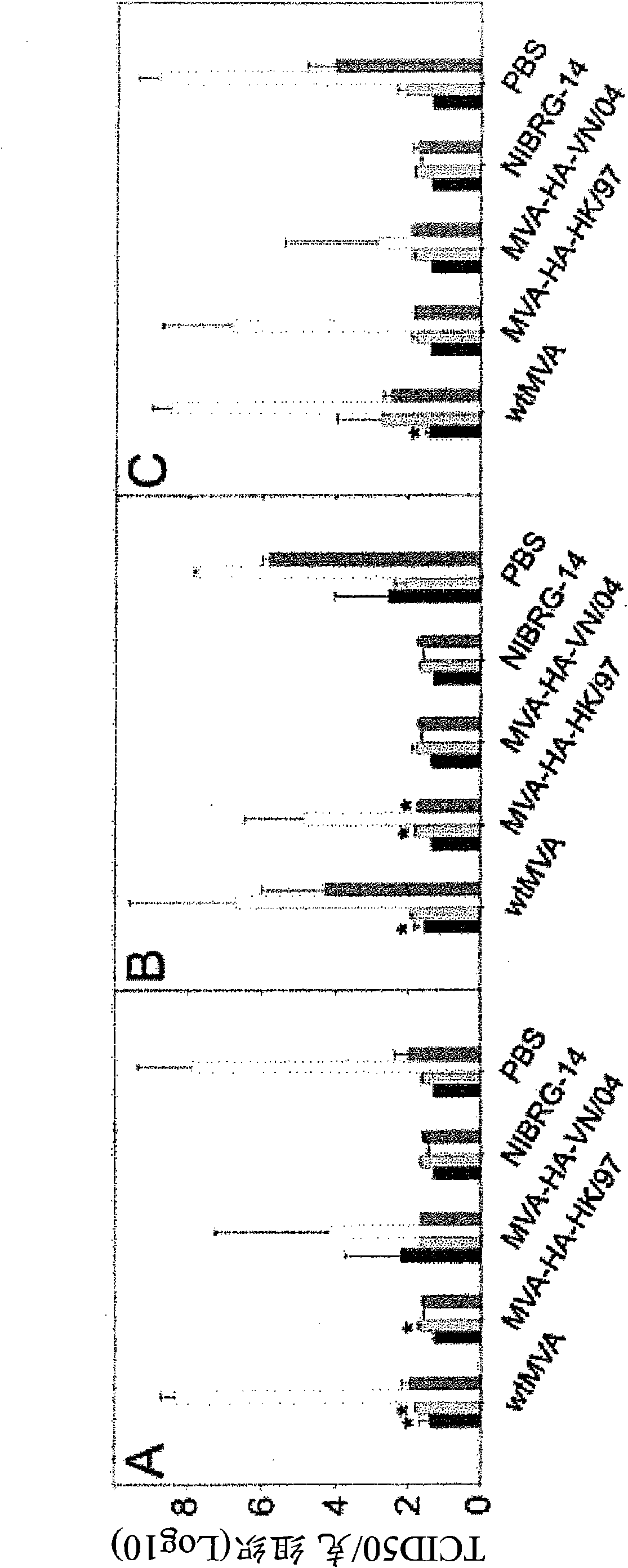
[0083] Preparation of the vaccine
[0084] The H5N1 virus was propagated in MDCK cells, and viral RNA was extracted from the culture supernatant using a high-purity RNA isolation kit (Roche, Almere, the Netherlands). Subsequently, cDNA was synthesized from vRNA using Superscript reverse transcriptase (Invitrogen, Carlsbad, California, USA) and 5'-AGCAAAAGCAGG-3' (SEQ ID NO. 9) oligonucleotide (Eurogentec, Seraing, Belgium) as a primer . Next, using Pfu (Pyrococcus furiosus, Stratagene, La Jolla, California, USA) as a thermostable DNA polymerase, the HA gene sequence was amplified by polymerase chain reaction. The primer sequence was extended using NotI and XhoI restriction sites to facilitate directional cloning to the cloning plasmid pBluescriptSK+ (Stratagene, La Jolla, California, USA).
[0085] The HA gene sequence was prepared from these plasmids by NotI / XhoI digestion, treated with Klenow polymerase to generate sticky ends, and cloned into the PmeI site of the MVA expressio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com