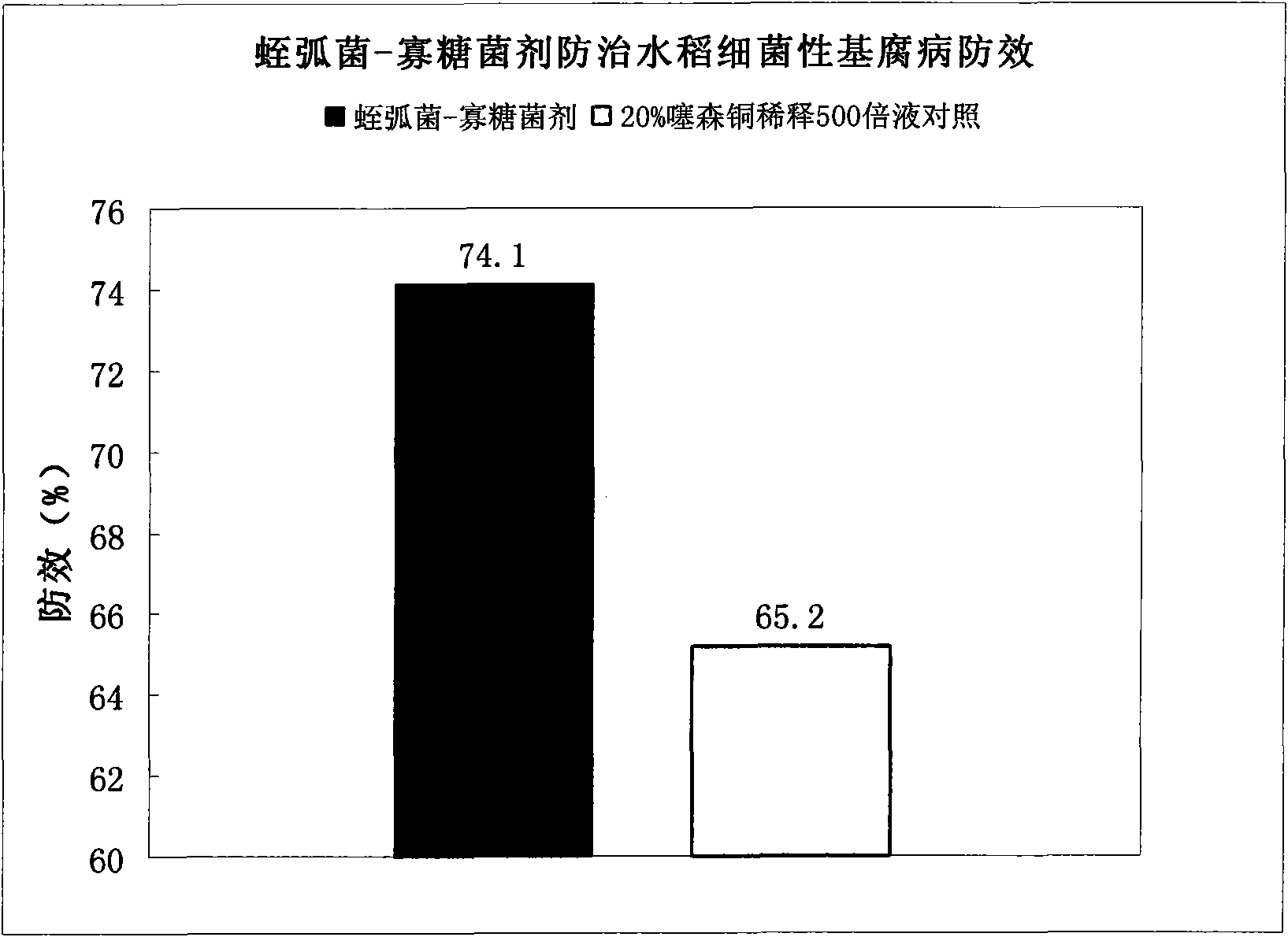
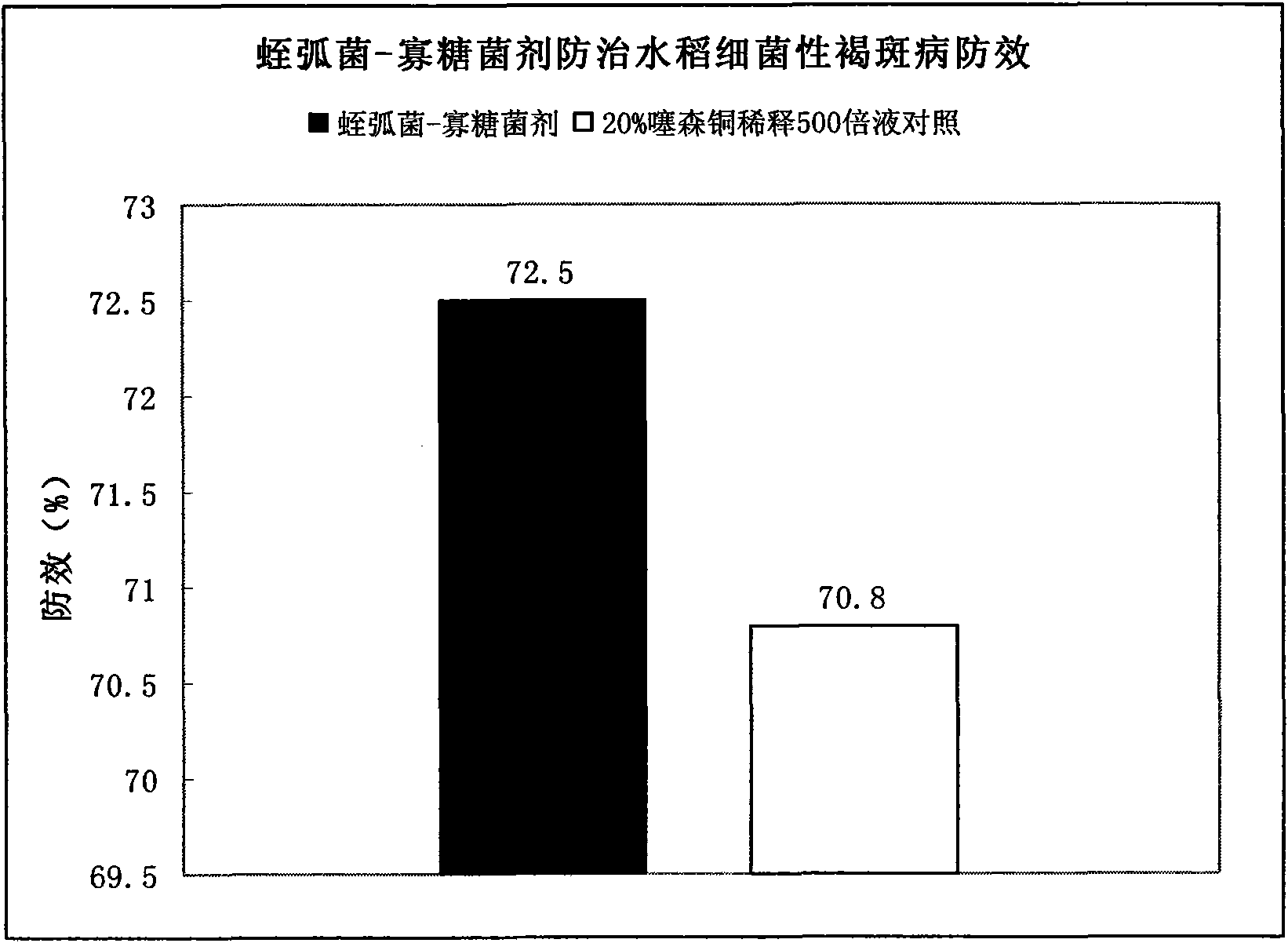
Bdellovibrio bacteriovorus for preventing and curing rice bacterial diseases and application thereof
A Bdellovibrio and bacterial technology, applied in the field of Bdellovibrio prevention and treatment of rice bacterial diseases, can solve the problems of no high-efficiency bio-control strain preparations, etc., to achieve excellent elimination ability, stable and long-lasting control effect, and rapid reproduction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Obtain Bdellovibrio BDFM05 by ultraviolet mutagenesis method
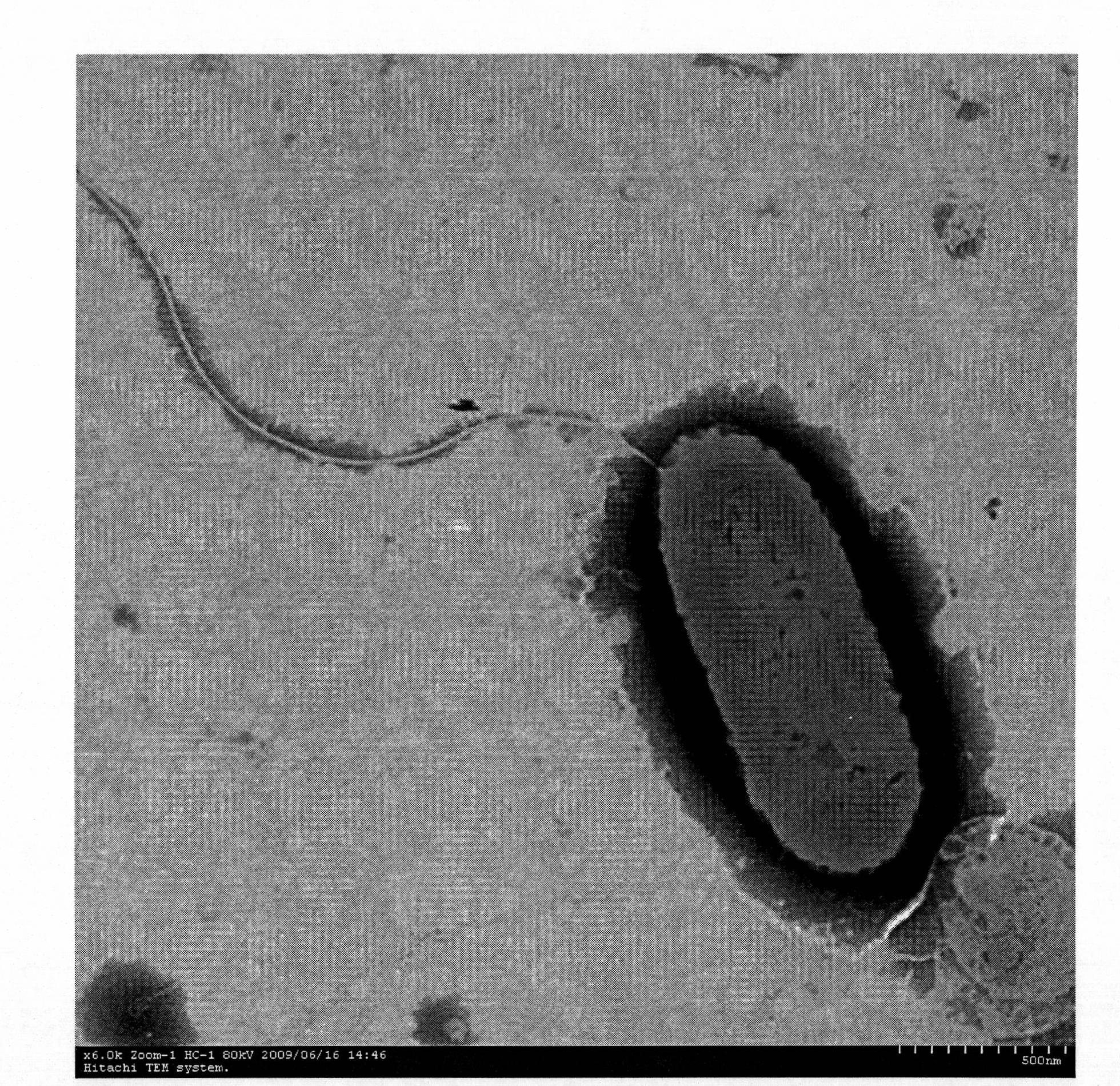
[0039] The starting strain is Bdellovibrio BDS02. BDS02 was isolated from mud samples in our laboratory, and was deposited in the Chinese Type Culture Collection Center of Wuhan University in Wuhan, China on August 7, 2009, with the preservation number CCTCC NO : M 209170; After negative staining, BDS02 was observed under the electron microscope, showing a single cell, arc-shaped, with a size of 0.65×0.2μm, terminal flagella, and the length of the flagella was at least 1.8μm; Cultivate at 28°C for four days to form transparent circular plaques with a diameter of 1-2mm;
[0040] According to the application number "2008100220488.3" of Bdellovibrio BDS02, the method in the national invention patent application titled "Ultraviolet-induced mutation in enhancing the lysis performance of Bdellovibrio" was used, and the power of the ultraviolet lamp was 15W, and the power of the auxiliary infrared light source ...
Embodiment 2
[0053] (1) Preparation of bacterial agents for preventing and treating bacterial diseases of crops
[0054] Inoculate Aeromonas hydrophila (Aeromonas hydrophila, purchased from the Culture Collection Center of Guangdong Institute of Microbiology, No. GIM1.172) in LB liquid medium, culture at 26 ° C for 20 h, and the culture liquid was centrifuged and concentrated at 7000 rpm for 10 min. The precipitate was added to the DNB liquid medium, inserted into Bdellovibrio BDFM05, cultured at 28°C for 40 hours, the culture solution was centrifuged at 8000rpm for 18min, and the supernatant was centrifuged at 16000rpm for 20min, and the precipitate was suspended with an appropriate amount of normal saline to prepare into a concentration of 10 8 pfu / L of Bdellovibrio swimming body bacteria liquid.
[0055] Add agar oligosaccharide to the obtained bdellovibrio swimming body bacterial liquid to make the concentration 20g / L, mix well, and obtain the preparation for preventing and treating b...
Embodiment 3
[0062] (1) Preparation of bacterial agents for preventing and treating bacterial diseases of crops
[0063] Inoculate Aeromonas hydrophila (Aeromonas hydrophila, purchased from the Culture Collection Center of Guangdong Institute of Microbiology, No. GIM1.172) in LB liquid medium, culture at 30 ° C for 20 h, and the culture liquid was centrifuged and concentrated at 5000 rpm for 20 min, then added Into phosphate buffer (0.2mol / L, pH7.2~7.6), insert Bdellovibrio BDFM05, culture at 28°C for 42 hours, the culture solution is the mixed bacterial solution of hiruplasma and swimming body, and the hiruplasma The concentration ratio of body and swimming body is 1:1, and the concentration of bacteria solution is 10 10 pfu / L.
[0064] Add chitosan oligosaccharide and agarose oligosaccharide to the obtained bacterial liquid to make the concentration respectively 15g / L, mix well, and obtain the preparation for preventing and treating bacterial diseases of rice.
[0065] (2) The bacteria...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com