Method for extracting and separating cesium ions from aqueous phase
A technology in cesium ion and water phase, which is applied in the field of nuclear fuel cycle and achieves the effect of good application prospect, improved extraction capacity and wide adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1, extracting Cs from neutral aqueous phase containing cesium ions +
[0019] When carrying out the cesium ion extraction experiment, take 0.5ml containing a certain amount of BPC6 (purchased from Tsinghua University Institute of Nuclear and New Energy Technology) C n mimNTf 2 Ionic liquid (purchased from the Green Chemistry Center of Lanzhou Institute of Chemistry and Physics, Chinese Academy of Sciences, website www.ionicliquid.org) and 0.5ml of aqueous phase containing a certain amount of cesium ions, adding tracer radioactivity to the system 134 Cs, fully mixed, oscillated, and centrifuged to separate phases, respectively take the upper and lower phases and measure the counts of the two phases with an automatic γ counter, thereby obtaining the extraction rate E or distribution ratio D.
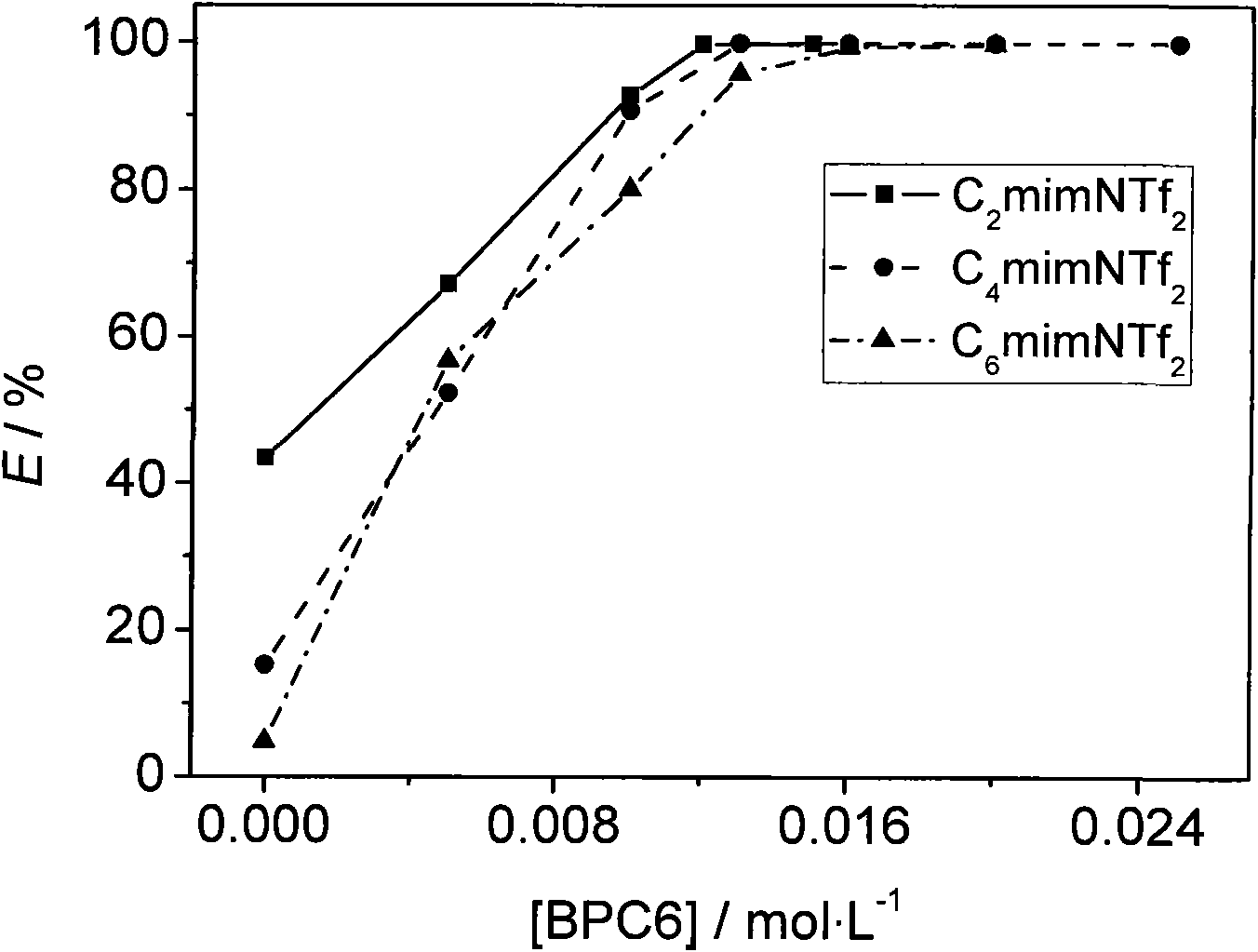
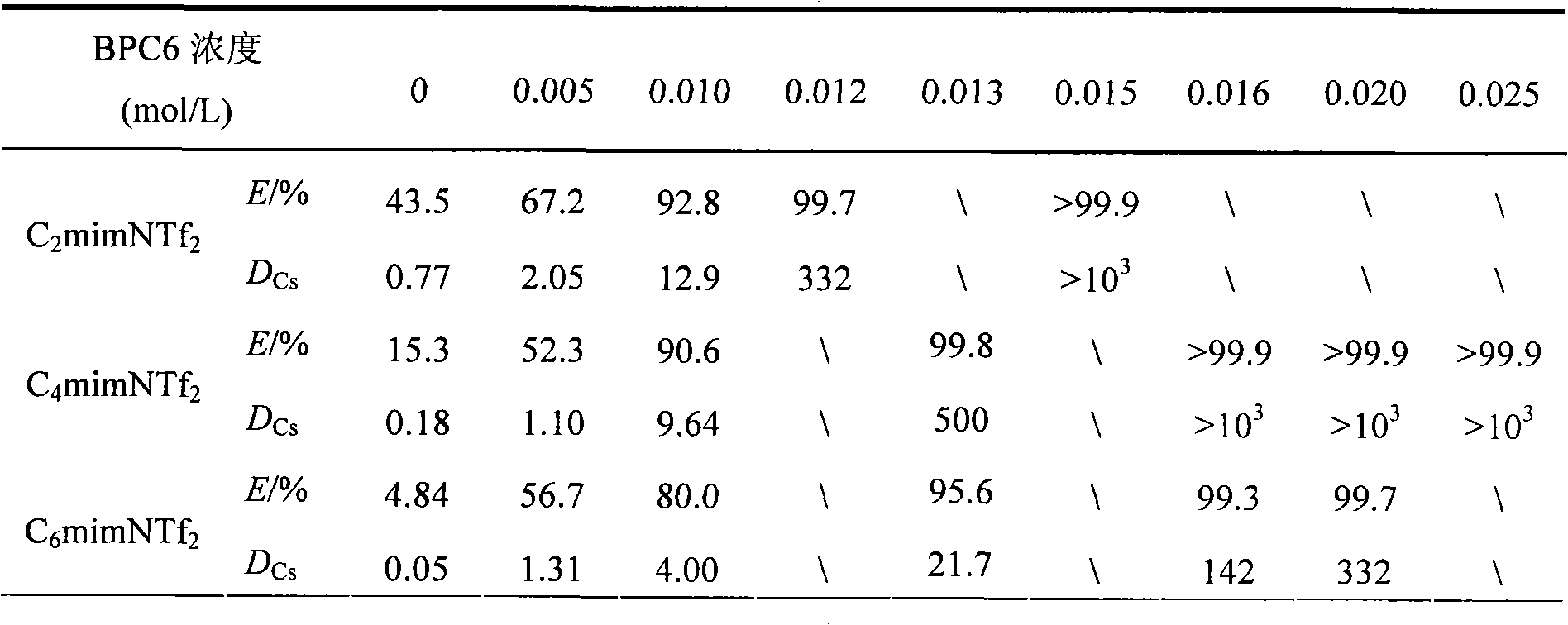
[0020] The cesium ion-containing aqueous phase adopted in the present embodiment is neutral, and Cs + The concentration is 0.01mol / L, without other interfering ions. ...
Embodiment 2
[0026] Example 2, extracting Cs from acidic aqueous phase containing cesium ions +
[0027] The aqueous phase containing cesium ions used in this example is acidic and does not contain other interfering ions.
[0028] Using C containing BPC6 concentration of 0.015mol / L n mimNTf 2 Ionic liquids (purchased from the Green Chemistry Center of Lanzhou Institute of Chemistry and Physics, Chinese Academy of Sciences, website www.ionicliquid.org) have different effects on Cs in aqueous phase with different acid concentrations. + ([Cs + ] is 0.01mol / L) to extract, and the results are shown in Table 2.
[0029] Table 2
[0030]
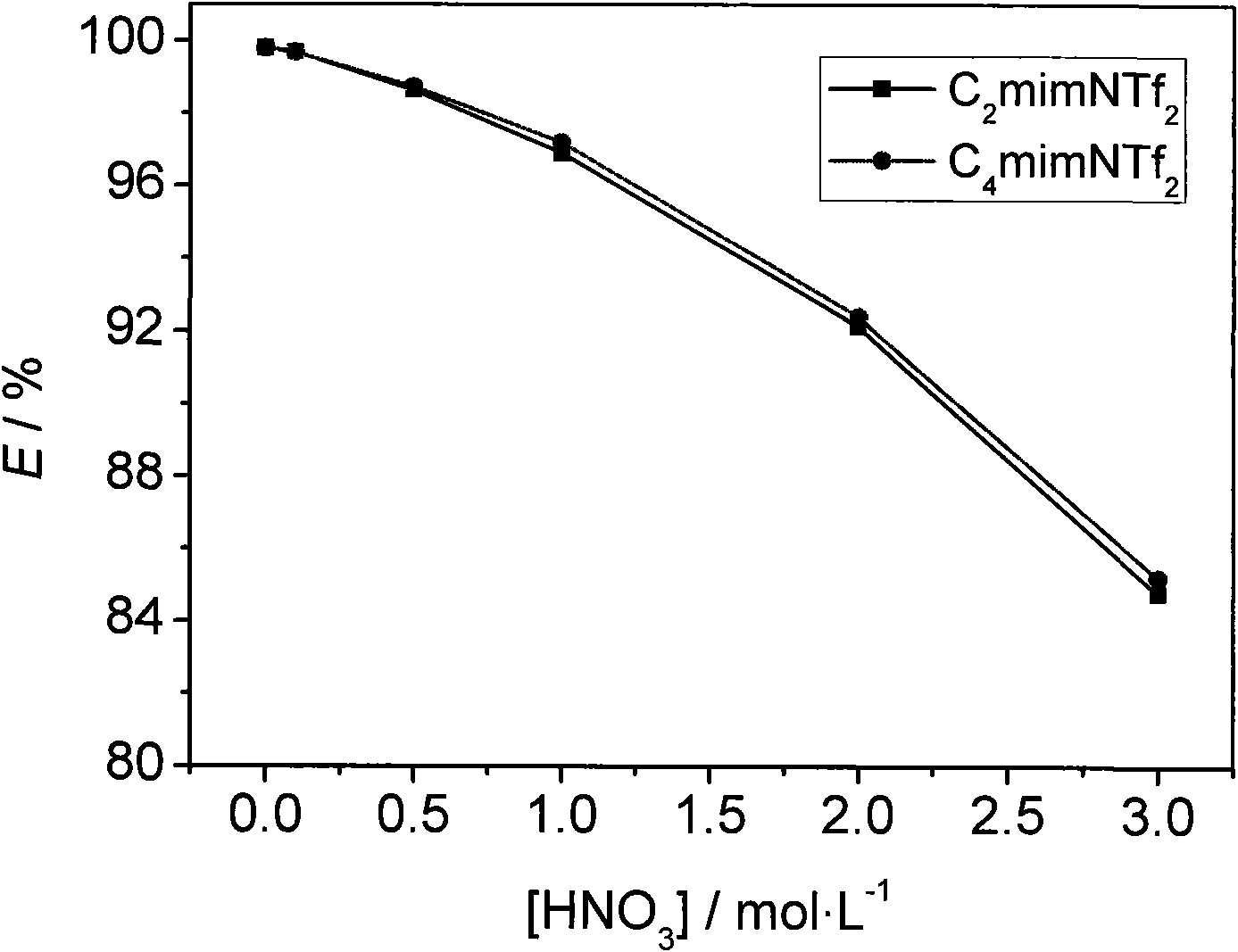
[0031] With the cesium ion extraction rate (E / %) in table 2 and the concentration of nitric acid in the aqueous phase, see figure 2 .
[0032] Depend on figure 2 It can be seen that the addition of nitric acid will make the system + The extraction performance is generally decreased. But even when the concentration of nitric acid in the aqueous ph...
Embodiment 3
[0033] Example 3, Extracting Cs from simulated high-level radioactive waste +
[0034] When carrying out the cesium ion extraction experiment, take 0.5ml containing a certain amount of BPC6 (purchased from Tsinghua University Institute of Nuclear and New Energy Technology) C n mimNTf 2 Ionic liquid (purchased from the Green Chemistry Center of Lanzhou Institute of Chemistry and Physics, Chinese Academy of Sciences, website www.ionicliquid.org) and 0.5ml of aqueous phase containing a certain amount of cesium ions and other ions or compounds, adding tracer radioactivity to the system 134 Cs, fully mixed, oscillated, and centrifuged to separate phases, respectively take the upper and lower phases and measure the counts of the two phases with an automatic γ counter, thereby obtaining the extraction rate E or distribution ratio D.
[0035] The cesium ion-containing aqueous phase adopted in this embodiment (simulated high-level waste liquid, wherein [HNO 3 ]=1.3mol / L) is shown in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com