New process for obtaining the crystalline form v of agomelatine
A technology of crystal form and compound, applied in the field of V crystal form of agomelatine or N-[2-ethyl]acetamide, can solve problems such as short storage stability period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
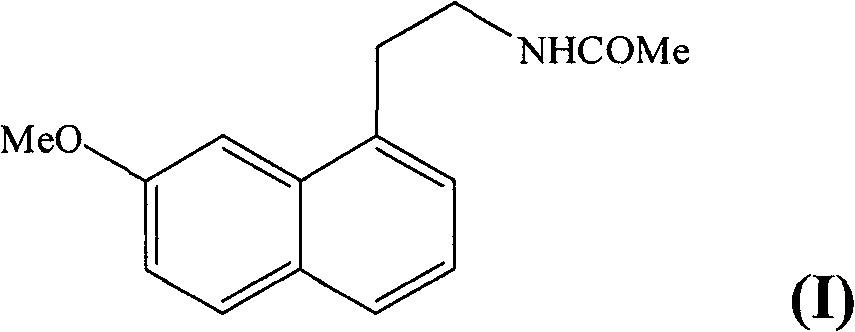
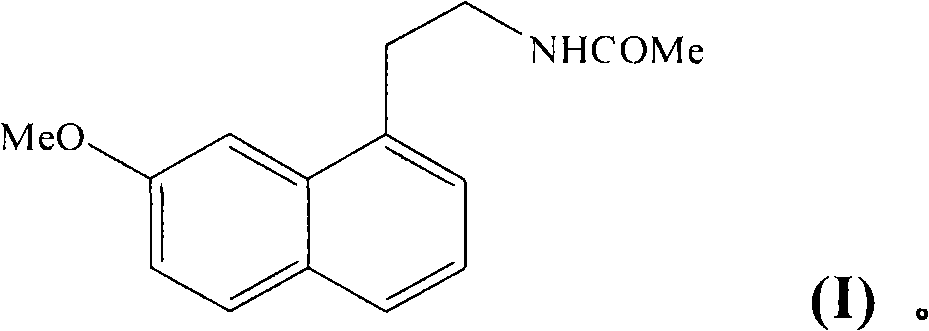
[0021] Example 1 : Form V of N-[2-(7-methoxy-1-naphthyl)ethyl]acetamide
[0022] A 10 g / l solution of agomelatine in an ethanol / isopropyl ether mixture (50 / 50: v / v) was introduced into the atomizer of a BUCHI 190 mini spray dryer. The inlet temperature of the drying chamber was 90°C and the outlet temperature was 66°C. The aerosolized powder was recovered in a collection bowl and identified using the following crystallographic data:
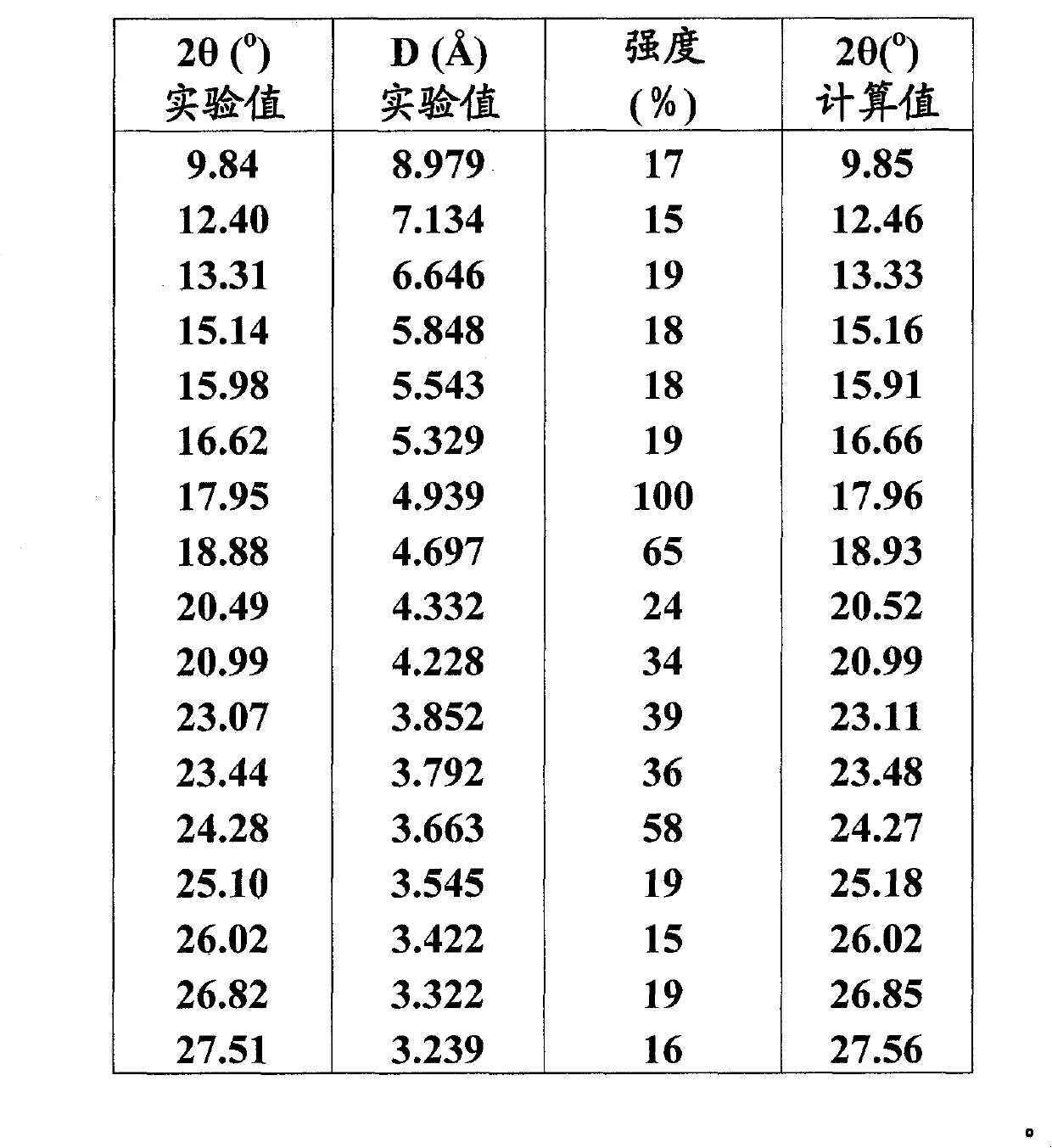
[0023] 1) Spectrum obtained with a Siemens D5005 diffractometer, using a 2θ angle range of 3°-30°, a span of 0.04° and 4 seconds per span:
[0024] - crystal structure of the unit cell: monoclinic,
[0025] -Unit cell parameter: a=11.967 b=17.902 c=15.423 β=124.5°
[0026] - Space group: P2 1 / n
[0027] - Number of molecules in the unit cell: 8 (Z'=2)
[0028] - the volume of the unit cell: V 晶胞 =2720.0
[0029] 2) The X-ray powder diffraction pattern below, which is measured with a Siemens D5005 diffractometer (copper to cathod...
Embodiment 2
[0031] Example 2 : Stability over time of crystal form V of N-[2-(7-methoxy-1-naphthyl)ethyl]acetamide obtained by nebulization
[0032] A 1 g sample of the compound obtained in Example 1 was placed under normal storage conditions: ambient pressure and temperature. After 21 months, the diffractogram of the obtained sample has not changed, it still has the characteristics of the obtained crystal form V.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap