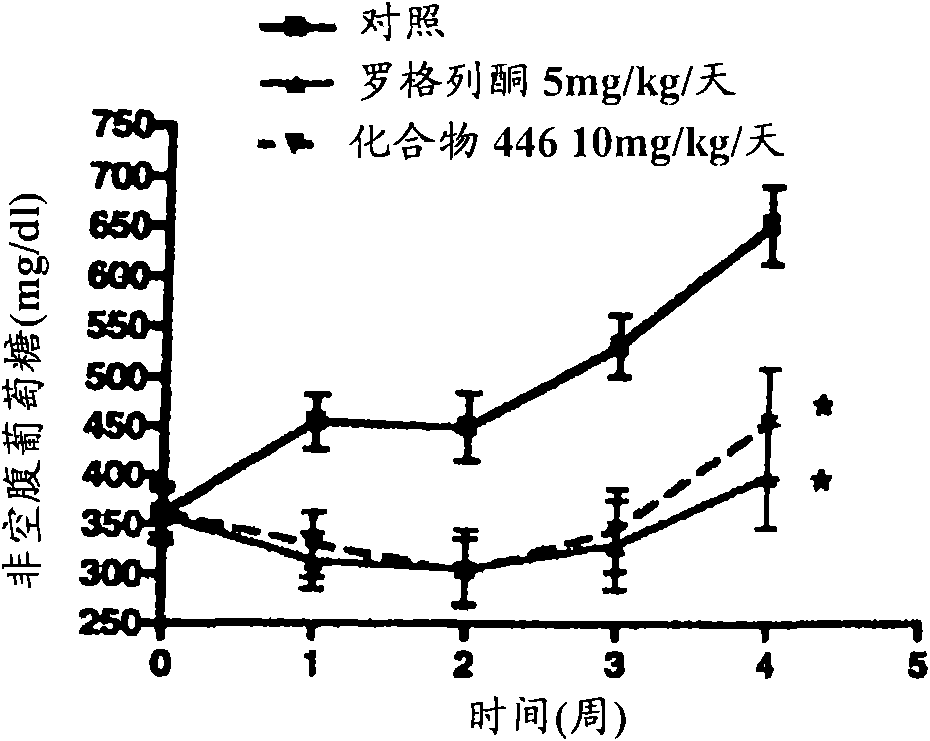
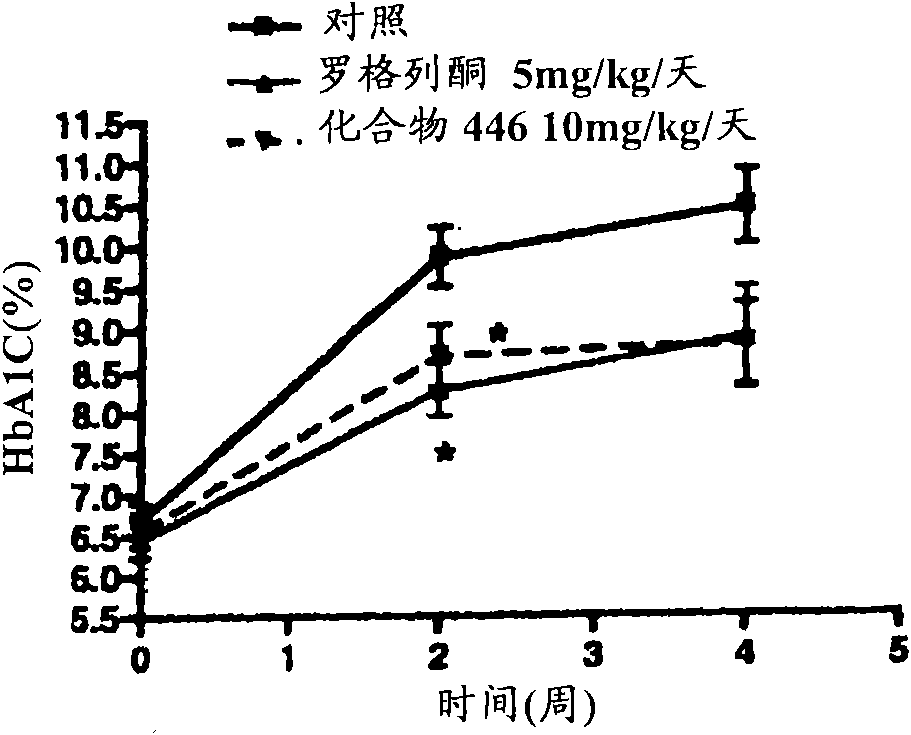
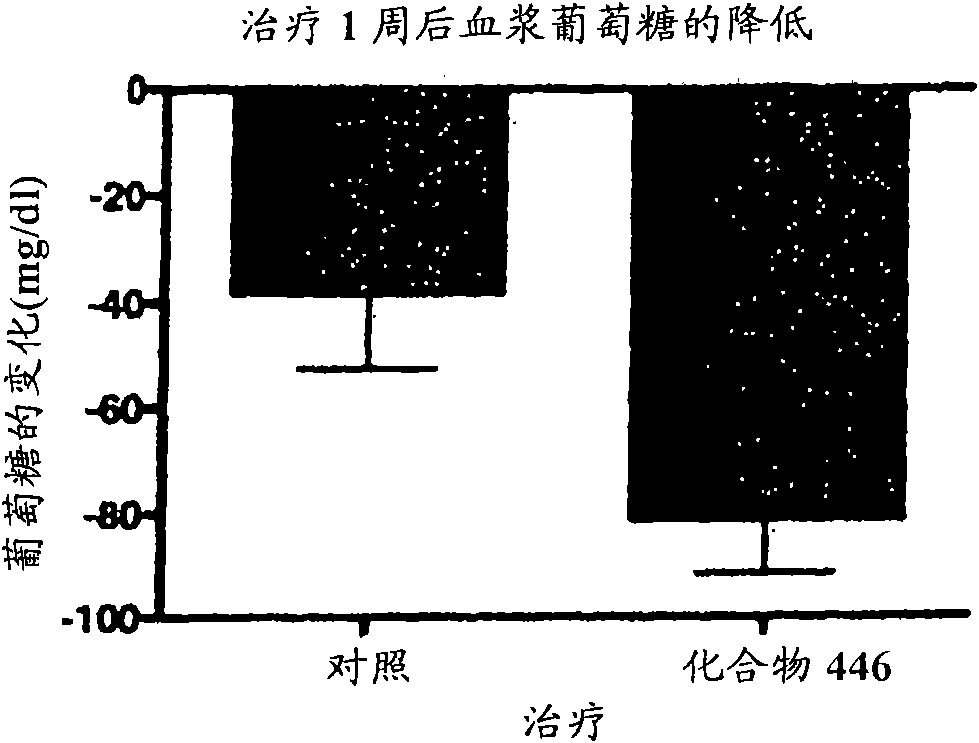
Piperidine derivatives and methods of use thereof
A diabetic, pyridyl-based technology applied in the field of piperidine derivatives, which can solve the problems of nausea and diarrhea, lactic acidosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
Embodiment 1
[0274] Synthesis of intermediate compound 5A
[0275] step 1
[0276]
[0277] To a solution of 10.81 g (100 mmol) of 2-amino-4-picoline in 250 mL of tert-butanol was added 26.19 g (120 mmol) of BOC anhydride. The reaction mixture was stirred overnight at room temperature, concentrated, dry-loaded onto silica gel, and subjected to flash chromatography (from 30% hexane / CH2 Cl 2 to 0-2% acetone / CH 2 Cl 2 ), yielding 15.25 g (73.32 mmol; 73%) of 1A as a white solid.
[0278] step 2
[0279]
[0280] To a solution of 1A (35.96 g, 173 mmol) in THF (1.4 L) at -78 °C was added 1.4 M BuLi in hexane (272 mL, 381 mmol) in portions over 30 min. The reaction mixture was then allowed to warm and stirred at room temperature for 2 hours, an orange precipitate formed. The mixture was cooled back to -78°C and pre-dried oxygen (through a Drierite column) was bubbled through the suspension over 6 hours while maintaining the temperature at -78°C. The color of the reaction mixtu...
Embodiment 2
[0291] Synthesis of intermediate compound 7A
[0292]
[0293] Compound 6A (42mmol), NBS (126mmol) and Bz 2 o 2 (4.2 mmol) of CCl 4 (400 mL) of the solution was refluxed at 80°C for 5 hours, cooled, and stirred at room temperature overnight. The reaction was filtered, concentrated, and the residue was purified by flash column (30% EtOAc / Hexanes) to afford target compound 7A (3.1 g, 23%).
Embodiment 3
[0295] Synthesis of intermediate compound 11A
[0296] step 1
[0297]
[0298] To a solution of 8A (10 g, 79.4 mmol) and DMAP (0.029 g, 0.24 mmol) in dichloromethane (150 mL) at 0 °C was added phthaloyl dichloride (16.1 g, 79.4 mmol) dropwise. The reaction mixture was stirred overnight at room temperature. After stirring overnight, the reaction was washed with saturated NaHCO 3 Washed with aqueous solution, water, dried and concentrated to give compound 9A (20 g, 99.8%) as a yellow solid, which was used directly without further purification.
[0299] step 2
[0300]
[0301] In a manner similar to that described in Example 2, compound 9A (20 g, 79.3 mmol) was converted to compound 10A.
[0302] step 3
[0303]
[0304] Compound 10A (0.5 g, 1.5 mmol) was mixed with hydrazine (0.5 M in ethanol, 5 mL, 2.5 mmol) and stirred at room temperature overnight. The reaction was diluted with water and extracted with dichloromethane. The organic layer was dried, co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com