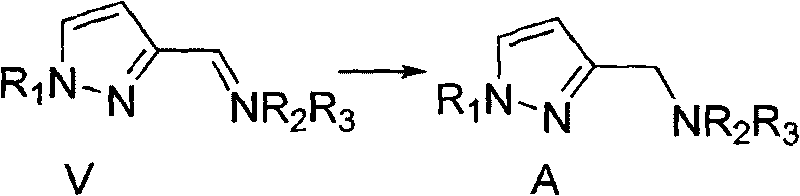N,N-substituent-1-(1H-pyrazole-3-yl) methylamine and preparation method thereof
A technology of pyrazole and methylamine, applied in the N field, can solve problems such as rare raw materials, and achieve the effects of easy availability of raw materials and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0043] The preparation of embodiment 14-(dimethylamino)-1,1-dimethoxybutyl-3-ene-2-one (formula II)
[0044] Mix 1,2-propanediketal (130g, 1.1mol) and 1,1-dimethoxy-N,N-dimethylmethylamine (134g, 1.1mol) and react at 100°C for 2h. After cooling, it was concentrated to give 4-(dimethylamino)-1,1-dimethoxybutyl-3-en-2-one (II) (144 g, 76%) as a red oil. 1 H NMR (CDCl 3 , 300MHz), 3.1 (d, 6H), 3.4 (s, 6H), 4.56 (s, 1H), 5.34 (d, 1H), 7.74 (d, 1H).
Embodiment 23
[0045] The preparation of embodiment 23-(dimethoxymethyl)-1H-pyrazole (formula III)
[0046] Add II (144g, 0.83mol) to hydrazine hydrate (83.2g, 1.66mol) and react at room temperature for 24h. Washed with saturated brine (50ml×3), extracted with ethyl acetate (40ml×3), dried over anhydrous sodium sulfate, concentrated to obtain 3-(dimethoxymethyl)-1H-pyrazole ( III) (113 g, 95%). 1 H NMR (CDCl 3 , 300MHz), δ7.58 (1H, d), 6.34 (1H, d), 5.62 (1H, s), 3.37 (6H, s).
Embodiment 31
[0047] The preparation of embodiment 31H-pyrazole-3-formaldehyde (formula IV)
[0048] Mix III (116g, 0.83mol), acetic acid (25g, 0.41mol) and water (100ml) and react at room temperature for 48h. Filter and dry to obtain the dimer of 1H-pyrazole-3-carbaldehyde as a yellow solid, add the dimer to pyrimidine (200ml) heated to 50°C and stir until completely dissolved to obtain 1H-pyrazole-3-carbaldehyde monomer solution (IV) (68 g, 87%). 1 H NMR (Pyridine-d 6 , 300MHz), δ10.38 (1H, t), 7.95 (1H, dd), 7.06 (1H, d).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap