Oral pharmaceutical composition of Fenofibrate
A fenofibrate and composition technology, applied in the field of hard capsule or soft capsule preparation, can solve the problems of incomplete dissolution, incomplete preparation method, and inability to lead to bioavailability of active ingredients and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
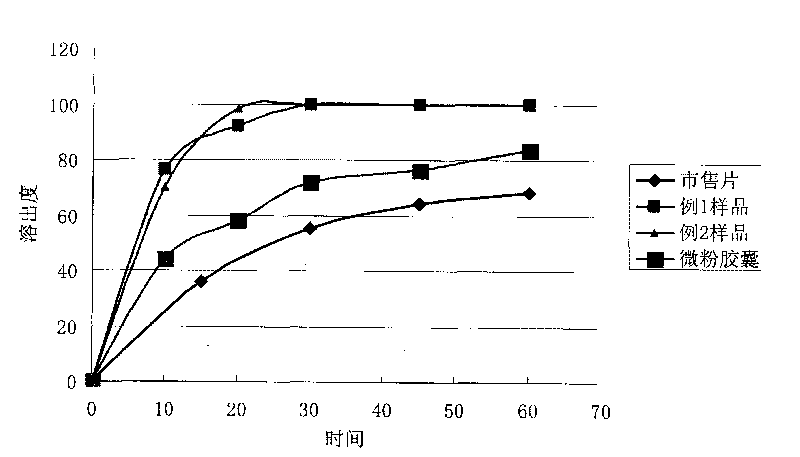
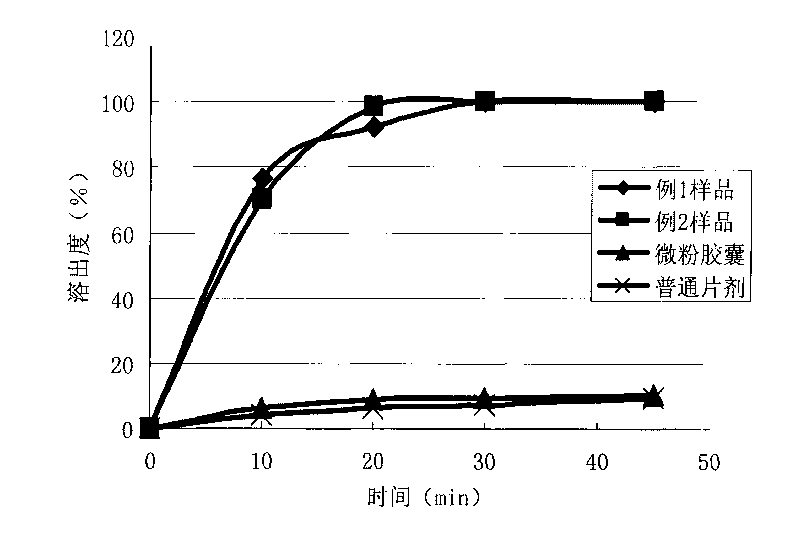
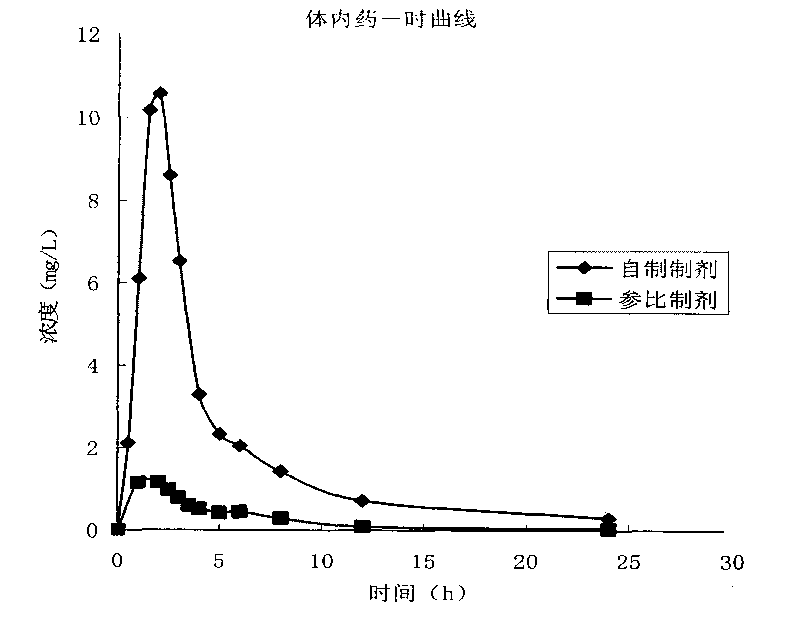
Image
Examples
Embodiment 1
[0046] Embodiment 1, the oral pharmaceutical composition of fenofibrate
[0047] Ingredient Amount
[0048] Fenofibrate 50mg
[0049] MCT 300mg
[0050] Oleic acid 100mg
[0051] EL-35 400mg
[0052] 1,2-propanediol 200mg
[0053] Mix the prescribed amount of MCT, oleic acid and EL-35 evenly to obtain a mixed solution, add the prescribed amount of fenofibrate to the mixed solution, stir and dissolve to obtain a transparent solution, and then add the prescribed amount of 1,2-propanediol , to obtain a transparent solution, which becomes the fenofibrate oral pharmaceutical composition of the present invention.
[0054] The above pharmaceutical composition is further divided into soft capsules or hard capsules, each equivalent to 50 mg of fenofibrate, that is, the pharmaceutical composition of the present invention is in the form of a unit dose of pharmaceutical preparations.
Embodiment 2
[0055] Embodiment 2, the oral pharmaceutical composition of fenofibrate
[0056] Ingredient Amount
[0057] Fenofibrate 50mg
[0058] MCT 100mg
[0059] Oleic acid 200mg
[0060] EL-35 500mg
[0061] PEG-400 200mg
[0062]According to the method similar to that described in Example 1, the MCT, oleic acid and EL-35 of the prescription amount are mixed uniformly to obtain a mixed solution, and the fenofibrate of the prescription amount is added to the mixed solution, stirred and dissolved to obtain a transparent solution , then add the prescribed amount of PEG-400 to obtain a transparent solution, and then divide the obtained transparent solution into soft capsules, each equivalent to 50 mg of fenofibrate, which is the pharmaceutical combination of the present invention in the form of unit dose soft capsules thing.
Embodiment 3
[0063] Embodiment 3, the oral pharmaceutical composition of fenofibrate
[0064] Ingredient Amount
[0065] Fenofibrate 10mg
[0066] MCT 125mg
[0067] Oleic Acid 175mg
[0068] EL-35 450mg
[0069] PEG-400 250mg
[0070] The above-mentioned components are processed in a similar manner to that described in Example 1 to obtain a transparent solution, and then the obtained transparent solution is divided into soft capsules, each equivalent to 10 mg of fenofibrate, which is in the form of a unit dose of soft capsules The pharmaceutical composition of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com