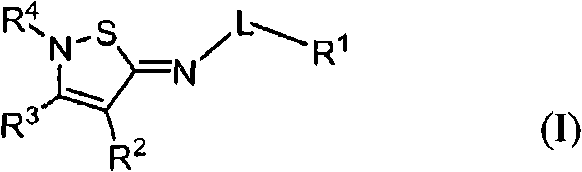
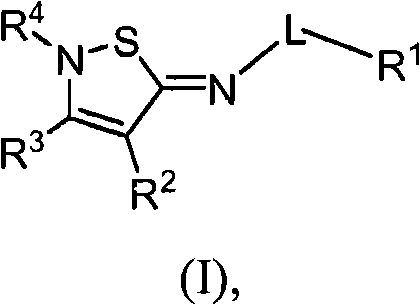
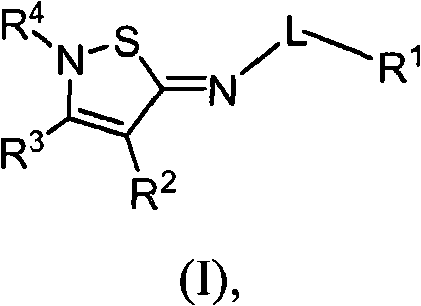
2-iminoisothiazole derivatives as cannabinoid receptor ligands
A technology of alkyl and alkynyl groups, applied in the field of compounds containing isothiazolylide groups, can solve the problems of ineffective treatment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0365] N-[(5Z)-4-butyl-2-tert-butylisothiazole-5(2H)-ylidene]-5-chloro-2-methoxybenzamide
Embodiment 1A
[0367] N-(tert-butyl)-N-hexylideneamine
[0368] CH 2 Cl 2 (10 mL) in tert-butylamine (5.25 mL, 50.0 mmol) and MgSO 4 (2 g) was slowly added hexanal (6.0 mL, 50 mmol). The reaction became exothermic halfway through the addition, so an ice bath was used briefly to control reaction rate and avoid solvent and amine boil-off. After the addition was complete, the reaction was stirred at room temperature for 2 h, filtered through celite under a stream of nitrogen, and washed with 20 mL of anhydrous CH 2 Cl 2 washing. The solvent was evaporated to obtain a pale yellow liquid. 1 H NMR (300MHz, CDCl 3 )δ0.87-0.91(m, 3H), 1.17(s, 9H), 1.25-1.35(m, 4H), 1.45-1.55(m, 2H), 2.23(m, 2H), 7.59(t, 1H) .
Embodiment 1B
[0370] 5-Chloro-2-methoxybenzoyl chloride
[0371] 5-Chloro-2-methoxybenzoic acid (11.3g, 60.56mmol) and SOCl 2 (9 mL, 123.7 mmol) was heated slightly in toluene (20 mL), during which a vigorous gas evolution occurred. After the gas evolution had subsided, the reaction was heated to reflux for 1.5 hours, cooled and stirred at room temperature overnight. The volatiles were evaporated in vacuo and the remaining material was treated with toluene and evaporated (2x) to remove excess SOCl 2 , a white solid was obtained which was directly used in the next step without purification. 1 H NMR (300MHz, CDCl 3 )δ3.92(s, 3H), 6.95(d, 1H), 7.53(dd, 1H), 8.03(d, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com