Tricyclene compounds with HIV antagonistic activity and preparation method and application thereof
A compound and solvent compound technology, which is applied in the field of tricycloalkene compounds, can solve problems such as rapid mutation of HIV virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
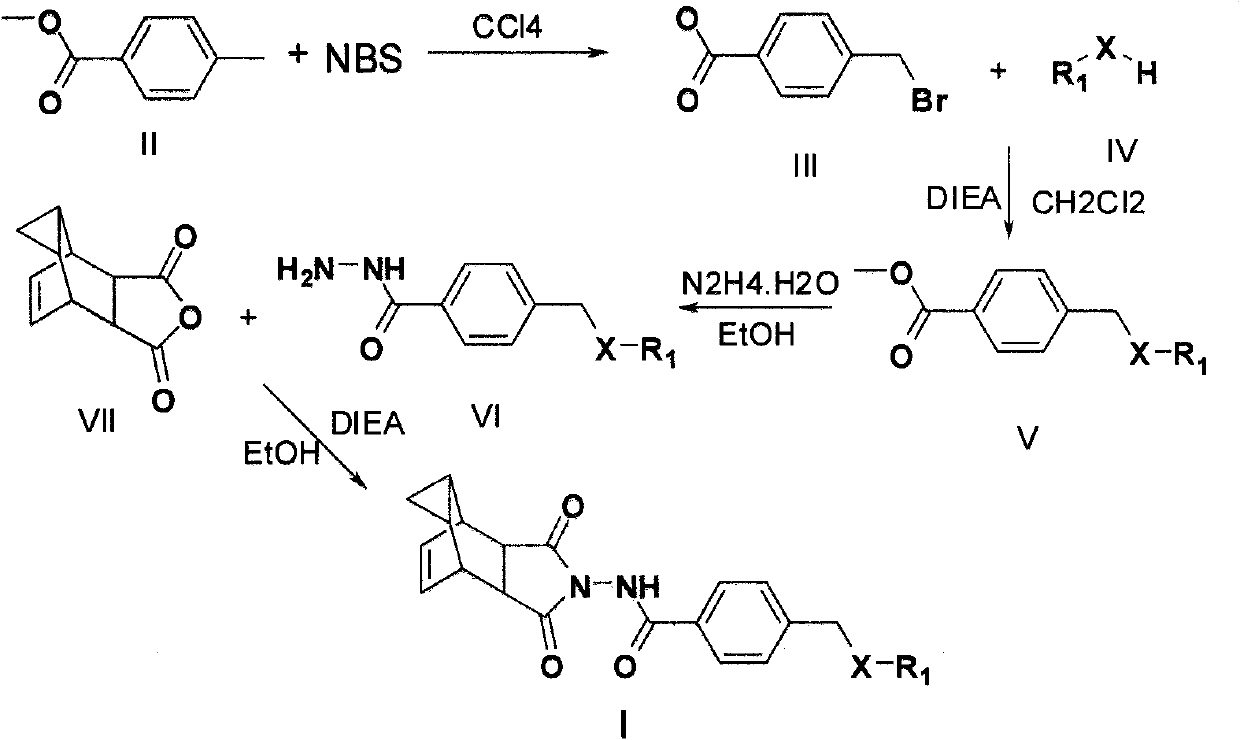
[0056] Compound 1: N-(3,3a,4,4a,5,5a,6,6a decahydro-1,3-dioxo-4,6-vinylidenecyclopropane[f]isoindole-2(1H )-yl)-4-(2-methylpiperazine-1-methyl)benzamide
[0057] Step 1: Methyl p-Bromomethylbenzoate
[0058] To a solution of methyl p-toluate (3.07g, 20mmol) in carbon tetrachloride (30ml), add N-bromosuccinimide (4.14g, 23mmol), and heat to reflux for 6h. Then the reaction solution was cooled, filtered, and washed three times with carbon tetrachloride (each 10ml), and the filtrate was collected and spin-dried to obtain yellow oily methyl p-bromomethylbenzoate (4.22g, yield 90%). Purification is required, and the next reaction can be carried out directly.
[0059] Step 2: Methyl 4-((4-methylpiperazin-1-yl)methyl)benzoate
[0060] To a solution of methyl p-bromomethylbenzoate (4.22g, 18mmol) and DIEA (5ml, 29mmol) in dichloromethane (35ml) was added dropwise with stirring 2.22ml (20mmol) of N-methylpiperazine. After the dropwise addition was completed, the reaction was carrie...
Embodiment 2
[0069] Compound 2: N-(3,3a,4,4a,5,5a,6,6a decahydro-1,3-dioxo-4,6-vinylidenecyclopropane[f]isoindole-2(1H )-yl)-4-((1 hydrogen-benzimidazol-1-yl)methyl)benzamide
[0070] The synthesis method is basically the same as that of compound 1, except that N-methylpiperazine in step 2) of the example is replaced with benzimidazole. Yield 60%, mp: 272-274°C.
[0071] The structural confirmation data are as follows:
[0072] 1 H NMR (400Hz, d 6 -DMSO): δ11.05 (s, 1H, CONH), 8.42 (s, H, imidazol-2-H), 7.85 (m, 2H, Ar-H), 7.67 (m, 1H, Ar-H), 7.42(m, 3H, Ar-H), 7.20(m, 2H, Ar-H), 5.78(s, 2H, CH=CH), 5.60(s, 2H, Ar-CH 2 ), 3.32(m, 4H, 2×COCH, 2× CH -CH=CH), 1.07(s, 2H, 2× CH -CH 2 ), 0.26 (m, 2H, CH 2 ).
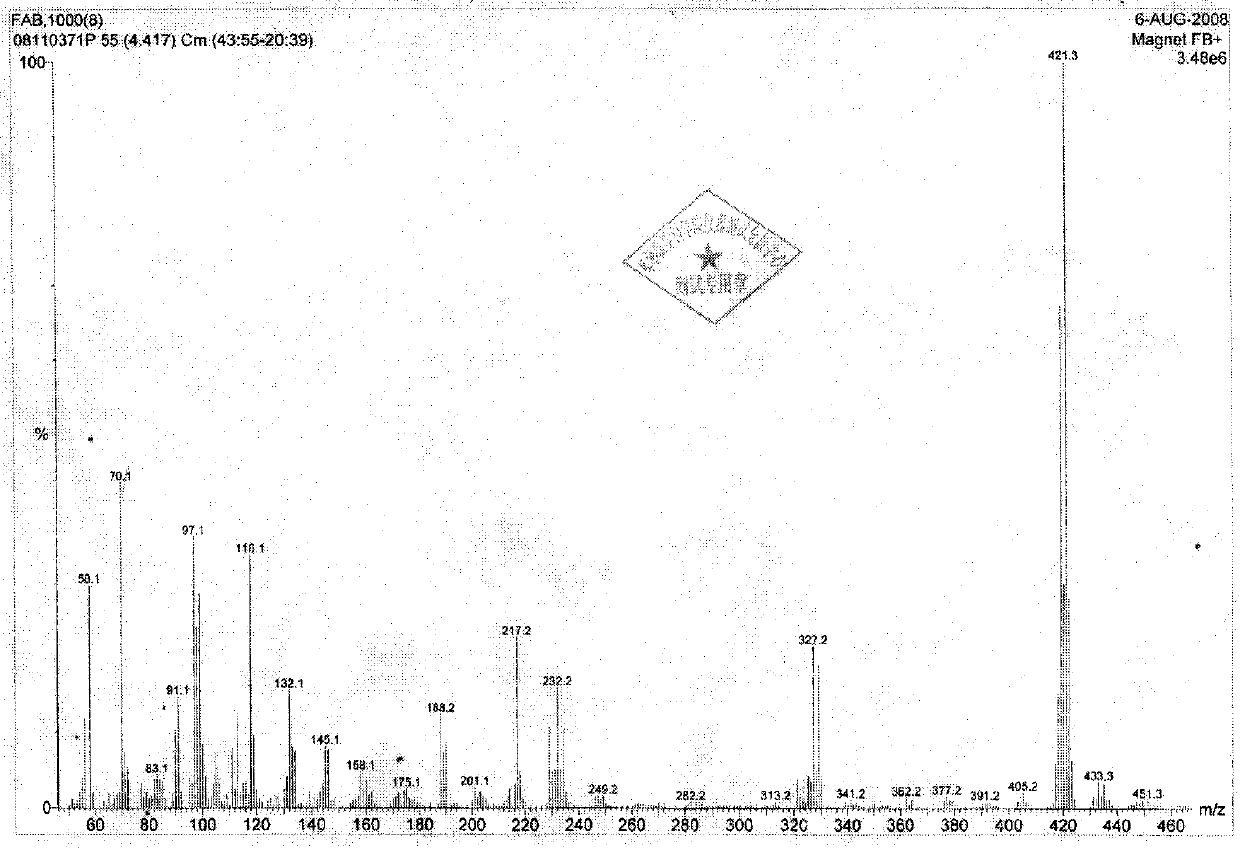
[0073] MS (FAB, Glycerin) m / z calculated value C 26 h 22 N 4 o 3 (M+1): 439.5, measured value: 439.2 (M+1).
Embodiment 3
[0075] Compound 3: N-(3,3a,4,4a,5,5a,6,6a decahydro-1,3-dioxo-4,6-vinylidenecyclopropane[f]isoindole-2(1H )-yl)-4-((2-methyl-1 hydrogen-benzimidazol-1-yl)methyl)benzamide
[0076] The synthesis method is basically the same as that of compound 1, except that the N-methylpiperazine in step 2) of the example is replaced by 2-methylbenzimidazole. Yield 74%, mp: 261-262°C.
[0077] The structural confirmation data are as follows:
[0078] 1 H NMR (400Hz, CDCl 3 ): δ10.62(s, 1H, CONH), 7.88(d, J=8.0Hz, 2H, Ar-H), 7.68(d, J=7.6Hz, 1H, Ar-H), 7.23(m, 3H , Ar-H), 6.94 (d, J=7.6Hz, 2H, Ar-H), 5.68 (s, 2H, CH=CH), 5.35 (s, 2H, Ar-CH 2 ), 3.43(s, 2H, 2×COCH), 3.12(s, 2H, 2× CH -CH=CH), 2.35(s, 3H, CH 3 ), 1.11 (d, J=4.4Hz, 2H, 2× CH-CH 2 ), 0.28 (m, 2H, CH 2 ).
[0079] MS(Turbo Spray)m / z calcd for C 27 h 24 N 4 o 3 Calculated: 453.5, Measured: 453.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com