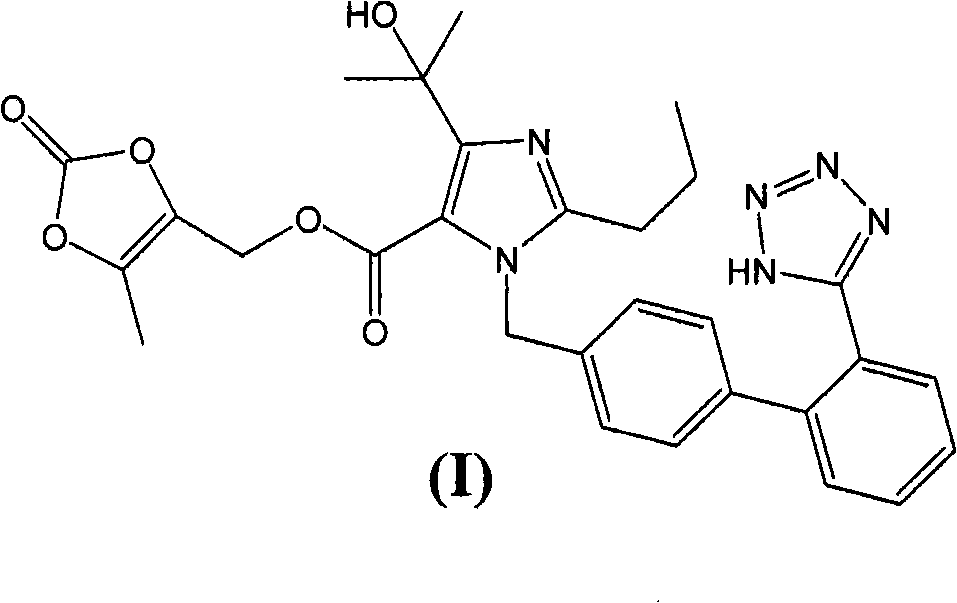
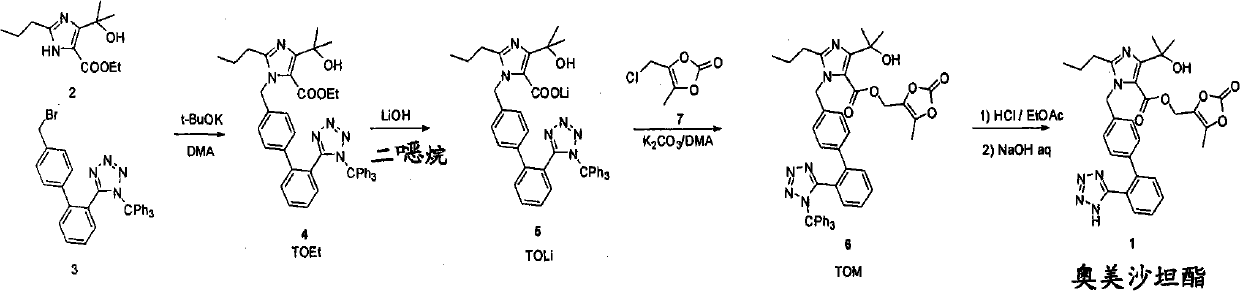
A process for the preparation of olmesartan medoxomil
A technology of olmesartan medoxomil and trityl olmesartan medoxomil, which is applied in the field of organic synthesis and can solve problems such as lack of experimental support
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1:-One-pot synthesis of trityl olmesartan medoxomil (6)
[0074] 4g (16.7mmol) 4-(1-hydroxyl-1-methylethyl)-2-propylimidazole-5-ethyl carboxylate (2), 9.28g (16.7mmol) 4-[2-(triphenyl Methyltetrazol-5-yl)phenyl]benzyl bromide (3) and 0.7 g (16.7 mmol) of lithium hydroxide hydrate were suspended in 70 mL of N,N-dimethylacetamide.
[0075] The reaction mixture was stirred at 50° C. for 3 hours, and then 1.05 g (25 mmol) of lithium hydroxide hydrate was added to the reaction mixture. The reaction mixture was stirred at 50 °C for a further 40 hours, then a third portion of lithium hydroxide hydrate (0.35 g, 8.33 mmol) was added and 3.84 g (24.6 mmol) of 4-chloromethyl-5-methyl was added in portions - 1,3-dioxol-2-one (7) (94%) in 10 mL of N,N-dimethylacetamide.
[0076] Completion of the reaction required further stirring of the reaction mixture at 50°C for 7 hours. To precipitate the product, the reaction mixture was poured into 400 mL of water and the resul...
Embodiment 2
[0077] Embodiment 2:-One-pot synthesis of trityl olmesartan medoxomil (6)
[0078] 4g (16.7mmol) 4-(1-hydroxyl-1-methylethyl)-2-propylimidazole-5-ethyl carboxylate (2), 9.28g (16.7mmol) 4-[2-(triphenyl Methyltetrazol-5-yl)phenyl]benzyl bromide (3) and 1.75 g (41.6 mmol) of lithium hydroxide hydrate were suspended in 70 mL of N,N-dimethylacetamide.
[0079] The reaction mixture was stirred at 50 °C for 46 h, then 2.53 g (18.32 mmol) of K 2 CO 3And 4.0 g (23.0 mmol) 4-chloromethyl-5-methyl-1,3-dioxol-2-one ( 7) (85%). The reaction mixture was stirred at 50 °C for an additional 5 hours to complete the reaction.
[0080] The product was precipitated from the reaction mixture by pouring it into 400 mL of a mixture of water and acetone (V / V=95 / 5). The resulting suspension was stirred overnight and the precipitate was filtered and washed with 200 mL of water. The wet cake was recrystallized in acetone to yield 9.95 g (75%) of trityl olmesartan medoxomil (6) (HPLC purity 97.7 ...
Embodiment 3
[0081] Embodiment 3:-One-pot synthesis of trityl olmesartan medoxomil (6)
[0082] 4g (16.7mmol) 4-(1-hydroxyl-1-methylethyl)-2-propylimidazole-5-ethyl carboxylate (2), 9.28g (16.7mmol) 4-[2-(triphenyl Methyltetrazol-5-yl)phenyl]benzyl bromide (3) and 0.7 g (16.7 mmol) of lithium hydroxide hydrate were suspended in 70 mL of N,N-dimethylacetamide. The reaction mixture was stirred for 45 minutes, then a further portion of lithium hydroxide hydrate (2.10 g, 50 mmol) was added to the reaction mixture. After stirring at 50°C for 48 hours, 4.9 g (31.0 mmol) of 4-chloromethyl-5-methyl-1,3-dioxa in 10 mL of N,N-dimethylacetamide was added in portions Cyclopenten-2-one (7) (94%). The reaction mixture was further stirred at 50 °C for 6 hours to complete the reaction. The product was precipitated from the reaction mixture by pouring it into 400 mL of a mixture of water and acetone (V / V=95 / 5). The resulting suspension was stirred overnight and the precipitate was filtered and washed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com