Sterile negative pressure isolation facility of capping machine
A negative pressure isolation and capping machine technology, applied in the field of isolation facilities, can solve the problems of capping room pollution, dust pollution, and dynamic monitoring of the cleanliness of the capping room is difficult to meet the standard, so as to achieve cleanliness standards and eliminate dust pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
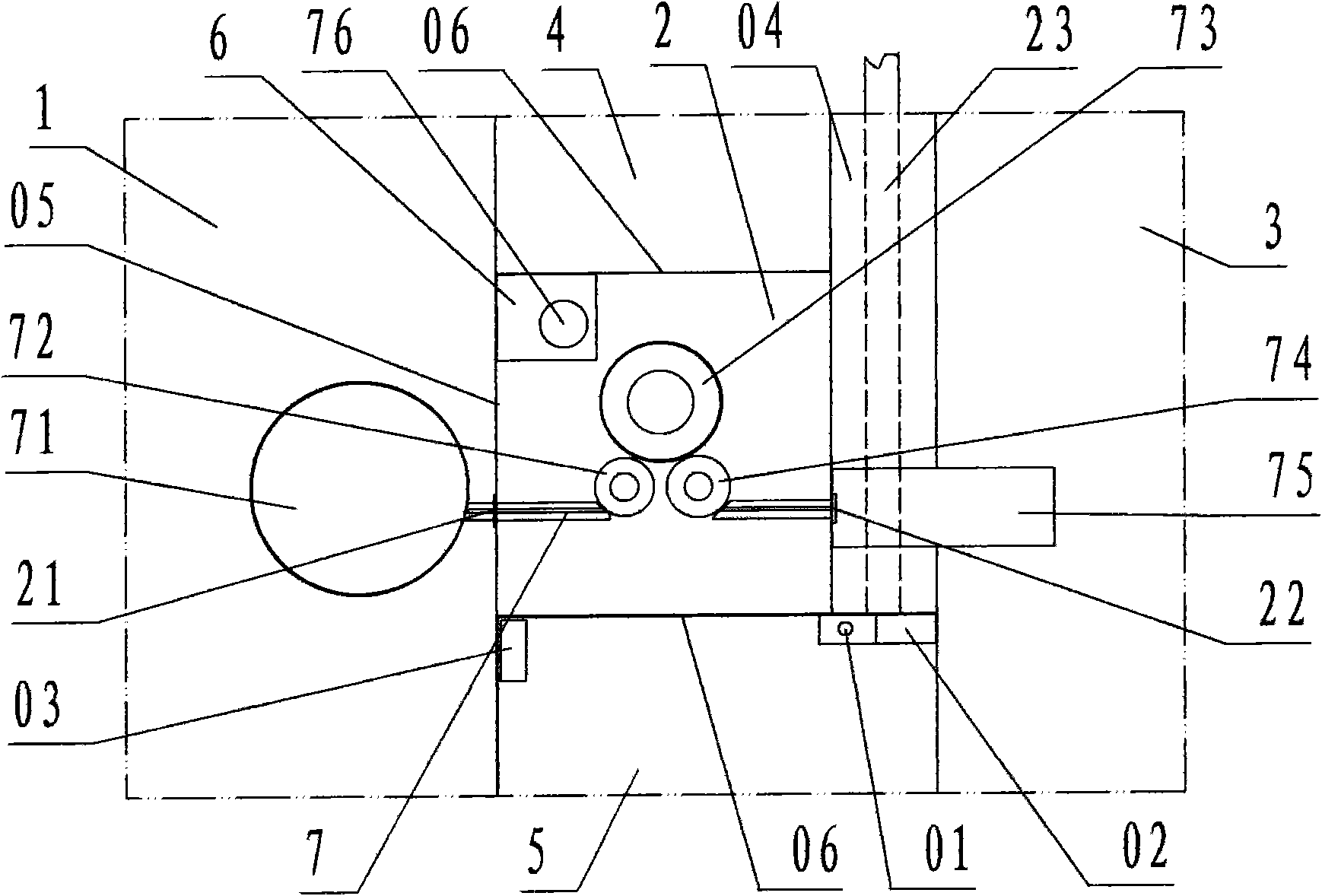
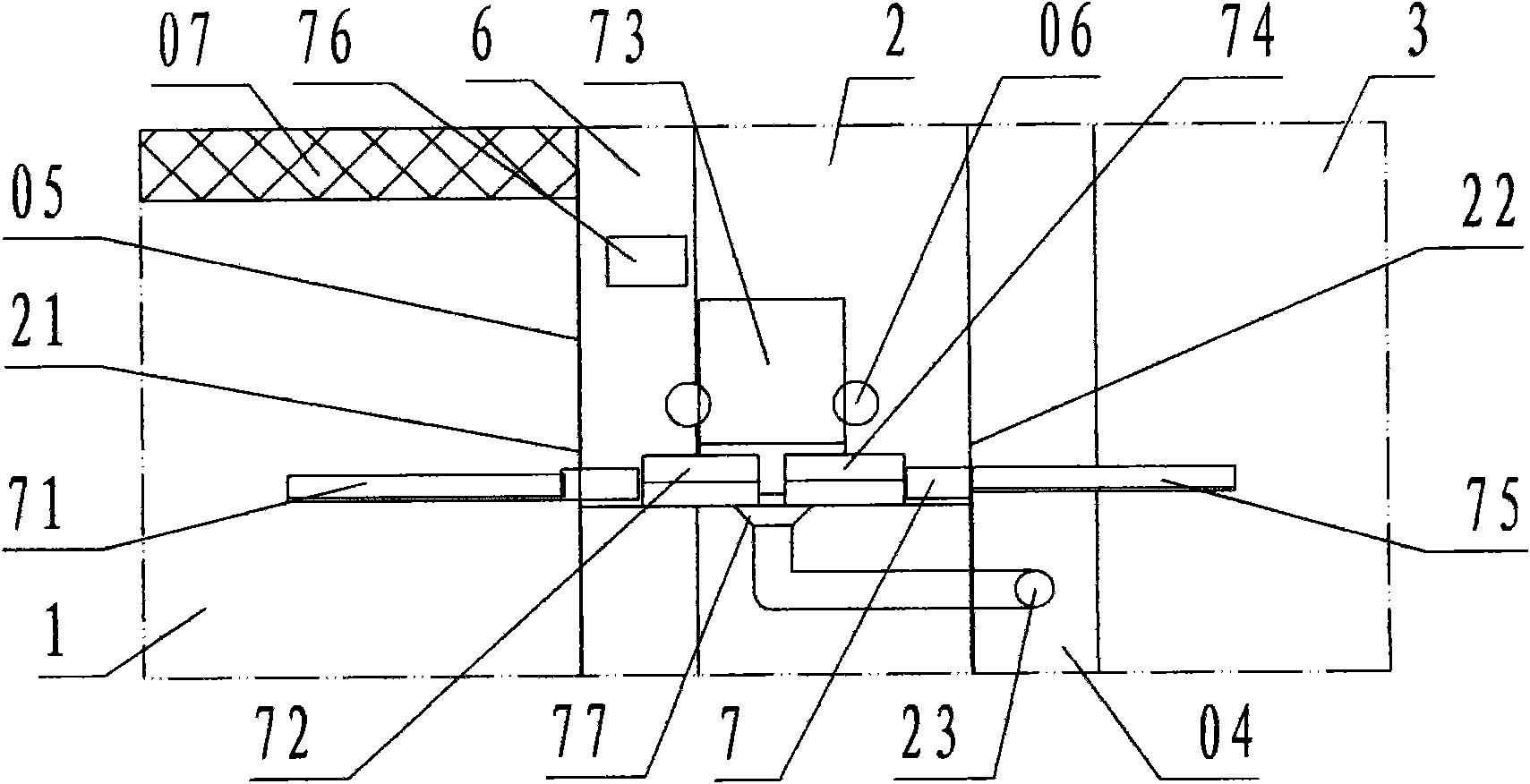
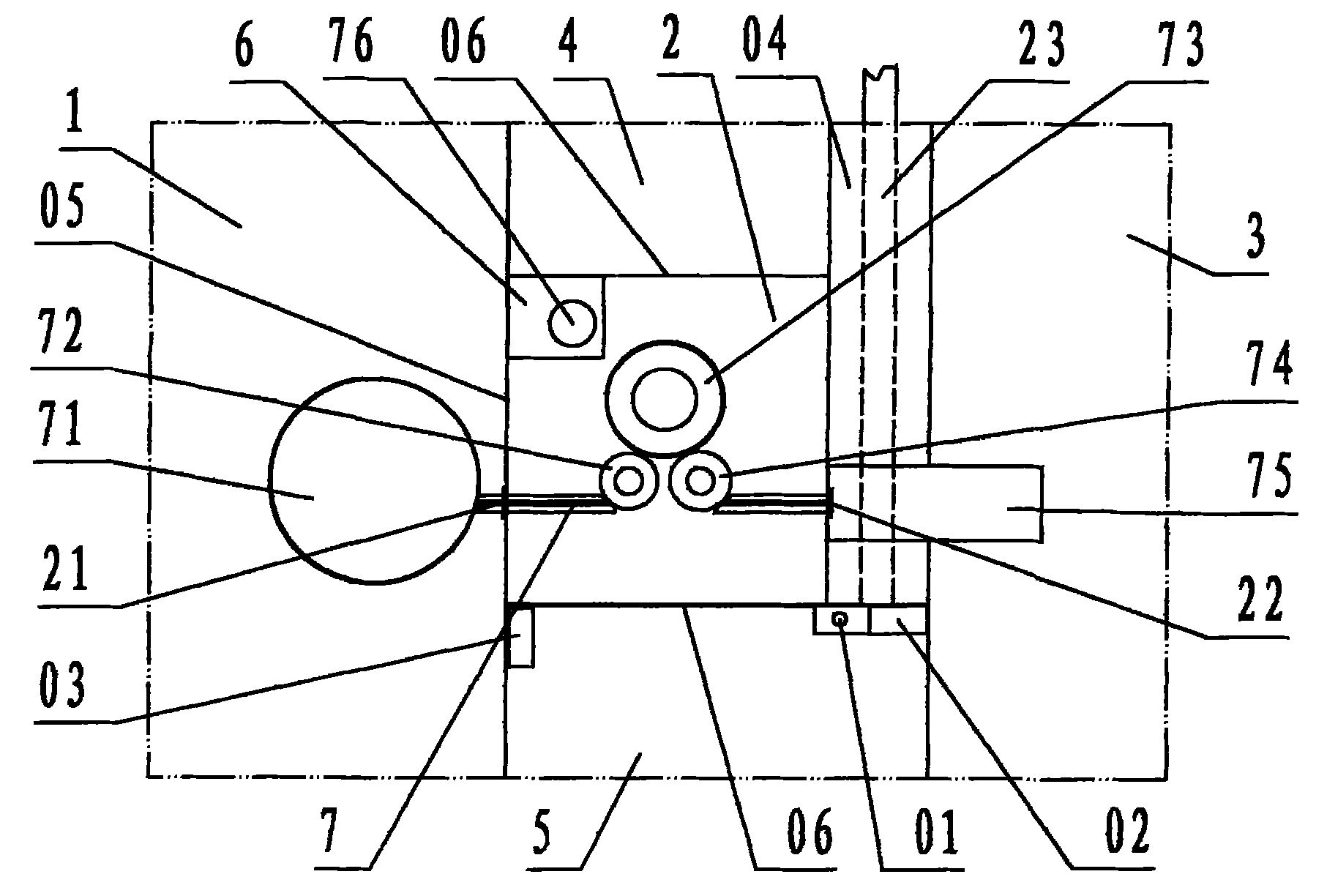
[0022] Refer to Figure 1 ~ Figure 2 , The aseptic negative pressure isolation facility of the capping machine of the present invention includes a filling room 1, a capping cabin 2, a bottle outlet room 3, an operation cabin 4, an operation cabin B 5, a transfer cabin 6, and a capping machine 7. Among them: the filling room 1, the capping room 2, the bottle outlet room 3, the operation cabin A 4, the operation cabin B 5, and the transfer cabin 6 are various workshops and cabins enclosed by transparent partition walls 05 ; According to the description of the left and right front and rear directions, the capping cabin 2 is the cabin for capping operations; the left side wall of the capping cabin is the filling room 1 for filling operations with filling equipment, and the left side wall of the capping cabin is provided There is a bottle inlet 21 communicating with the filling room 1, and the bottle inlet is equipped with a valve; the right side wall of the capping cabin is the bot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com