Sterile negative pressure isolation facility of capping machine
A negative pressure isolation and capping machine technology, applied in the field of isolation facilities, can solve the problems of pollution between capping, dust pollution, rubber plug loosening, etc., achieve cleanliness standards, and eliminate dust pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
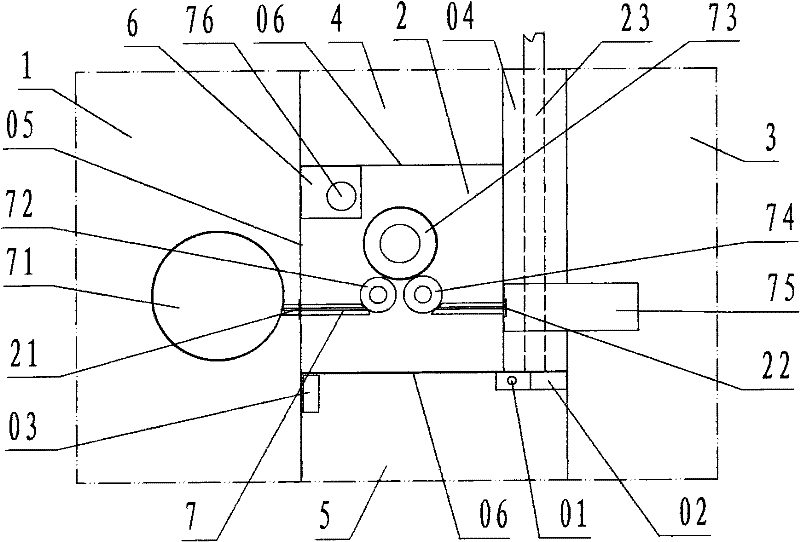
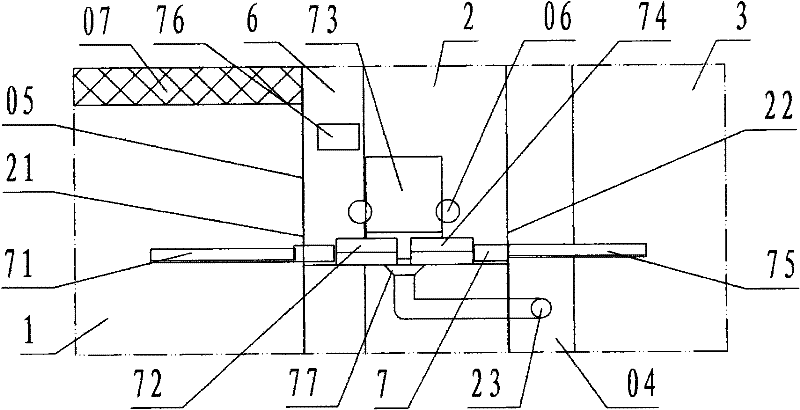
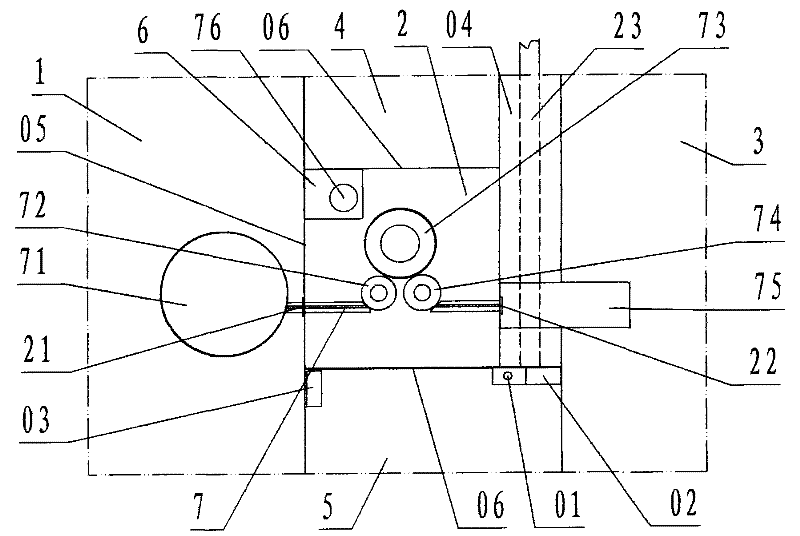
[0022] refer to Figure 1 ~ Figure 2 , a sterile negative pressure isolation facility for a capping machine of the present invention, comprising a filling room 1, a capping cabin 2, a bottle outlet room 3, a operating cabin 4, a second operating cabin 5, a transfer cabin 6, and a capping machine 7, wherein: the filling room 1, the capping cabin 2, the bottle outlet room 3, the A operation cabin 4, the B operation cabin 5, and the transfer cabin 6 are each work workshop and cabin enclosed by a transparent partition wall 05 ; According to the description of the left and right front and rear directions, the capping cabin 2 is the cabin of the capping operation; the left side wall of the capping cabin is the filling room 1 of the filling operation provided with filling equipment, and the left side partition wall of the capping cabin is provided with There is a bottle inlet 21 communicating with the filling room 1, and the bottle inlet is provided with a valve; the right next door ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com