New method for continuously operating to synthesize capecitabine
A technology for synthesizing cards and new methods, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of cumbersome operation, large solvent consumption, and many times of distillation recovery.
Inactive Publication Date: 2010-08-25
江苏吴中苏药医药开发有限责任公司
View PDF0 Cites 12 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
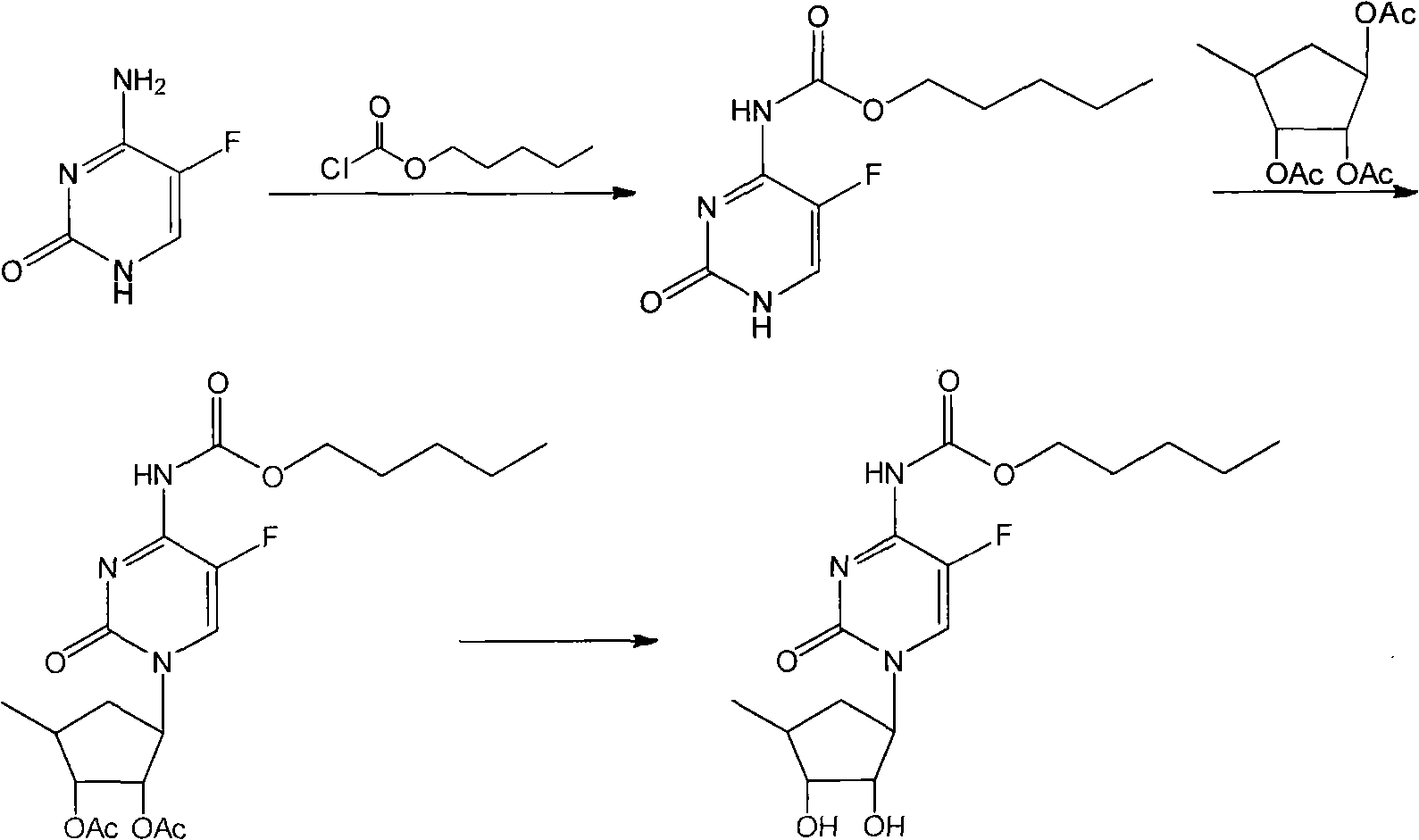
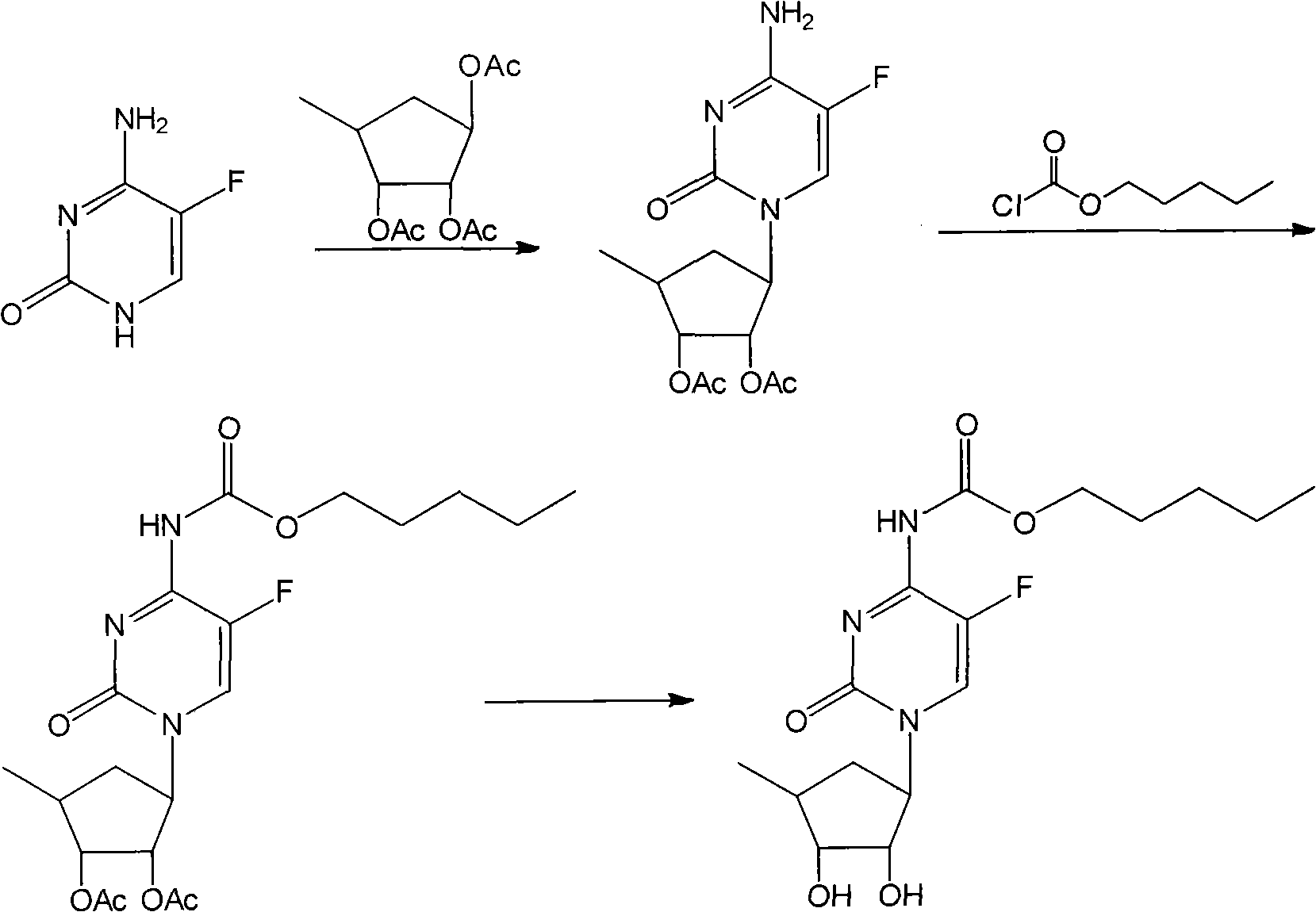
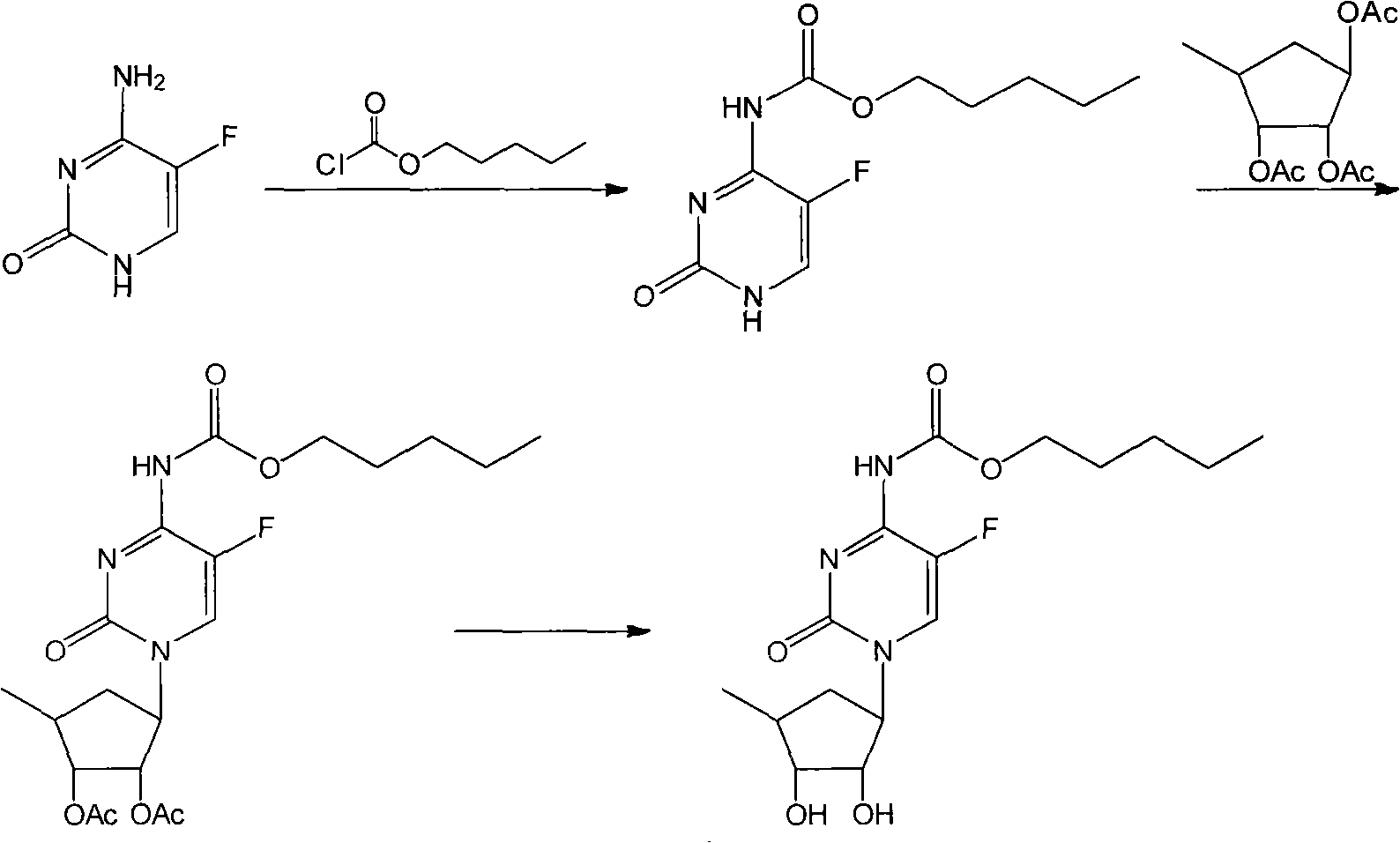
However, in the literature, the three-step reaction is operated separately, and the separation of two intermediates is required. The loss is relatively large, the yield is low, the operation is cumbersome, the amount of solvent is large, the number of distillation recovery is large, and the cost is high.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
example 1
example 2
example 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention relates to a new method for continuously operating to synthesize capecitabine, which is characterized in that 5-flucytosine is subjected to acylation, condensation and hydrolysis to synthesize the capecitabine. The invention has the advantages of reasonable reaction sequence, continuous operation of three steps, operation simplification, high yield, low cost and less pollution.
Description
technical field The invention belongs to the technical field of medicinal chemistry, and in particular relates to a new continuous operation method for synthesizing capecitabine. Background technique There are several synthetic methods for the important anticancer drug capecitabine, the most commonly used one is the method using 5-fluorocytosine as the starting material. According to the different reaction sequences, this method is divided into two types: method one: Reference: US 5742949 US 2005 137392 WO 2008 145403 US 2009 209754 Since the price of 1,2,3-triacetyl-5-deoxy-D-ribose is much higher than other raw materials, the first step of this method is to use 1,2,3-triacetyl-5-deoxy-D-ribose, Therefore, the consumption is higher and the cost is increased, which is the disadvantage of this method. Method Two: references WO 2005 080351 WO 2009 071726 In the second step of this method, 1,2,3-triacetyl-5-deoxy-D-ribose is used, and the dosage is reduced,...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07H19/06C07H1/00
Inventor 武卫唐磊朱晶晶张荣久曹庆先
Owner 江苏吴中苏药医药开发有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com