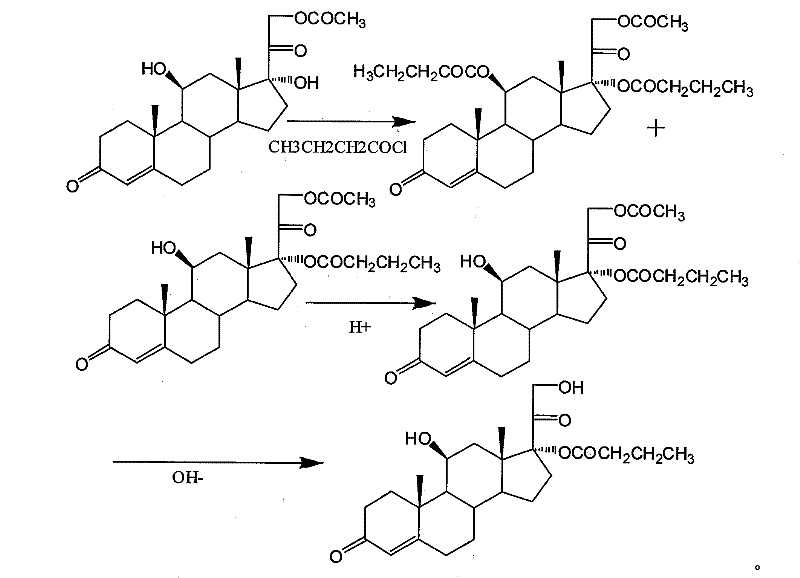
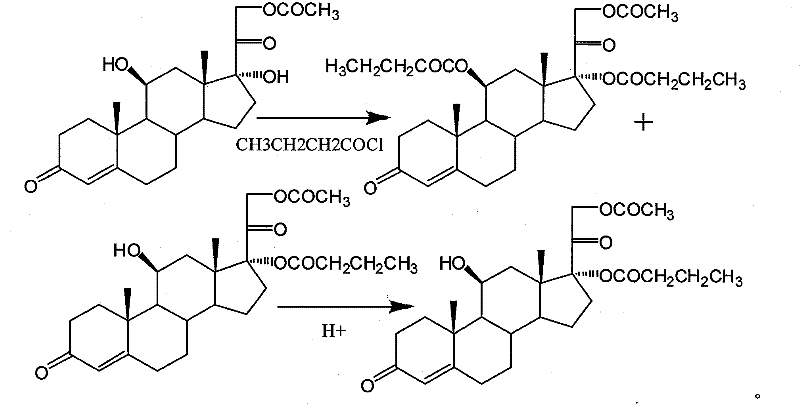
Synthesis of hydrocortisone butyrate
A technology of hydrocortisone acetate and hydrocortisone, which is applied in the fields of steroids and organic chemistry, and can solve the problems of product impurity, increased refining times, and failure to obtain high purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Acylation:
[0049]Take 1mmol of hydrocortisone acetate and place it in 10ml of dichloromethane, cool the resulting solution to 0-5°C, and add 4ml of triethylamine and 0.05mmol DMAP at this temperature, and then put it under the condition of 0-5°C slowly add 1.5 mmol of butyryl chloride, then stir the resulting mixture at 0°C, react for 3 hours, add hydrochloric acid to adjust the pH to 2, stir for 10 minutes, wash with water until neutral, and then wash with dichloromethane 10ml×3 The aqueous phase was extracted and the organic phase was combined, concentrated, poured into methanol for recrystallization, cooled and filtered to obtain 0.86 mmol of hydrocortisone 17α-butyrate-21-acetate (acylate).
[0050] Before hydrochloric acid was added to the reaction, samples were taken and analyzed by HPLC to find that the ratio of hydrocortisone 17α-butyrate-21-acetate to 11β, 17α-bisbutyrate-acetate hydrocortisone was 1:0.16.
[0051] In the product obtained after the reaction,...
Embodiment 2
[0055] Acylation:
[0056] Take 1 mmol of hydrocortisone acetate and place it in 10 ml of chloroform, cool the resulting solution to 0-5 °C, and add 4 ml of pyridine and 0.1 mmol of pyridine hydrobromide at this temperature, and then Under the condition of ℃, slowly add 1.3 mmol of butyryl chloride, then stir the obtained mixture under the condition of 0 ℃, add hydrochloric acid to adjust to 2 after reacting for 3 hours, after stirring for 10 minutes, wash with water until neutral, and then wash with dichloromethane 10ml ×3 times extract the aqueous phase and combine the organic phases, concentrate, pour into methanol for recrystallization, and obtain 0.83 mmol of hydrocortisone 17α-butyrate-21-acetate (acylate).
[0057] hydrolysis:
[0058] Dissolve the acylate in dichloromethane, add an equal amount of methanol, pass N 2 For protection, add 0.1g of potassium carbonate dissolved in methanol. After the reaction is complete at -20°C, the reaction solution is neutralized to n...
Embodiment 3
[0060] Acylation:
[0061] Take 1 mmol of hydrocortisone acetate and place it in 10 ml of dichloromethane, cool the resulting solution to 0-5 ° C, and add 4 ml of pyridine and 0.05 mmol DMAP at this temperature, and then at a temperature of 0-5 ° C, Slowly add 1.3mmol of butyryl chloride, then stir the resulting mixture at 0°C, add acetic acid to adjust to 5 after reacting for 3 hours, stir for 10 minutes, wash with water until neutral, and then extract the water with 10ml of dichloromethane×3 times phase, the organic phases were combined, concentrated, poured into methanol for recrystallization, and obtained 0.9 mmol of hydrocortisone 17α-butyrate-21-acetate (acylate).
[0062] Before hydrochloric acid was added to the reaction, samples were taken and analyzed by HPLC to find that the ratio of hydrocortisone 17α-butyrate-21-acetate to 11β, 17α-bisbutyrate-acetate hydrocortisone was 1:0.19.
[0063] In the product obtained after the reaction, the ratio of hydrocortisone 17α-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com